Abstract
Background
There have been several reports describing the capability of ginseng extracts as an adjuvant. In this study, we tested if ginsan, a polysaccharide extracted from Panax ginseng, was effective in enhancing antibody response to orally delivered Salmonella antigen.
Methods
Ginsan was treated before oral salmonella antigen administration. Salmonella specific antibody was determined by ELISA. mRNA expression was determined by RT-PCR. Cell migration was determined by confocal microscopy and flow cytometry. COX expression was detected by western blot.
Results
Ginsan treatment before oral Salmonella antigen delivery significantly increased both secretory and serum antibody production. Ginsan increased the expression of COX in the Peyer's patches. Various genes were screened and we found that CCL3 mRNA expression was increased in the Peyer's patch. Ginsan increased dendritic cells in the Peyer's patch and newly migrated dendritic cells were mostly found in the subepithelial dome region. When COX inhibitors were treated, the expression of CCL3 was reduced. COX inhibitor also antagonized both the migration of dendritic cells and the humoral immune response against oral Salmonella antigen.
Most of the currently available vaccines are licensed for use only via a nonmucosal route, usually involving subcutaneous or intramuscular inoculation (1). Parenteral vaccines elicit predominantly systemic immune responses that are usually unable to prevent the initial interaction step between the pathogen and the host (2). As the most effective means of inducing an immune response at a specific effector site is local stimulation at a related inductive site, oral vaccination represents one of the most promising vaccination strategy to prevent gastrointestinal infections.
Polysaccharides isolated from botanical sources (mushrooms, algae, lichen and higher plants) have attracted a great deal of biomedical attention because of their broad spectrum of therapeutic properties and relatively low toxicity (3,4). Among numerous plant polysaccharides, ginseng is a slow-root growing herb that has been used medicinally for more than 3,000 years by practitioners of oriental medicine (5). There have been several reports describing the immune adjuvant capability of ginseng extracts. Immunization using porcine parvovirus vaccines utilizing single purified ginsenosides as an adjuvant stimulates the potent production of antibody, whose titer matches or exceeds that produced from the use of vaccines administered with aluminum hydroxide as the adjuvant (6). Ginseng extract is a well-tolerated adjuvant, and displays a superior adjuvant effect when used for immunization against Staphylococcus aureus in dairy cattle (7). Thus, the use of ginseng as a co-adjuvant provides a simple, well tolerated and inexpensive alternative for improving the potency of aluminum hydroxide-based vaccine adjuvants (8). In a clinical study, ginseng significantly reduced the frequency of influenza or common cold, and natural killer cell activity levels were nearly twice as high in the ginseng group than in the placebo group (9).
Ginsan is a soluble polysaccharide extracted from the roots of Panax ginseng (10). Various functions of ginsan as biologic modifiers have been reported (10-15). Recently, we have reported that ginsan can modulate mucosal immune response in asthma model (16) and bacterial infection (17). However, its possibility as a mucosal vaccine adjuvant has not been studied. In this study, we studied the potential of ginsan as an adjuvant for oral immunization using paraformaldehyde fixed Salmonella typhimurium as an antigen.
BALB/c mice were purchased from KOATECH (DaeJeon, Korea). Coral green fluorescent protein (cGFP) transgenic mice in a BALB/c background were kindly provided by Dr. Kwon (International Vaccine Institute, Seoul, Korea). The mice were subsequently bred at Chonnam National University in chambers maintained at 22~23℃ with equal 12 h periods of light and dark each day. The Cyclooxygenase (COX)-1-specific inhibitor SC-560 (Cayman Chemical, Ann Arbor, MI, USA) and the non-specific COX-1 inhibitor acetylsalicylic acid (ASA; Sigma-Aldrich, St. Louis, MO, USA) dissolved in ethanol were used. TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA) and primers were obtained from Bionics (Seoul, Korea). All experiments using mice were followed the guideline approved by the Committee for the Care and Use of Laboratory Animals at Chonnam National University.
The ginsan polysaccharide was purified from the ethanol insoluble fraction of the aqueous P. ginseng extract as described previously (15). Further purification was carried out successively using size exclusion and ion exchange column chromatography. Nuclear magnetic resonance analysis revealed that the purified fraction was composed of α(1→6) glucopyranoside and β(2→6) fructofuranoside in a 5:2 molar ratio. The endotoxin level in the purified ginsan preparation was less than 0.03 EU/mg as measured using the Endosafe™ Limulus amebocyte lysate assay (Charles River Laboratories, Wilmington, MA, USA) according to the manufacturer's instructions. The purified ginsan was dissolved in phosphate buffered saline (PBS; pH 7.4) and filtered through a 0.22µm Millipore membrane (Millipore, Billerica, MA, USA).
Ginsan (100 mg/kg) was intraperitoneally introduced to the mice once a day for two days before oral antigen administration. COX inhibitors, SC-560 (5 mg/kg) and ASA (100 mg/kg), were orally administered on the same day ginsan treatment was done (16). To continuously suppress COX, orally vaccinated mice were fed with indomethacin (30µg/ml) dissolved in drinking water.
Salmonella typhimurium were grown to late log phase in LB broth at 37℃ with vigorous aeration. For immunization, S. typhimurium was fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min and washed three times with sterile PBS. Mice fasted overnight were orally immunized with 1×109 colony forming units of S. typhimurium suspended in 500 µl PBS using oral zonde needle. Mice were immunized twice during a two week interval.
Samples were taken from each group of immunized mice. Stool samples collected by isolating each mouse in a dark box for 10 minutes before other procedures. Blood samples were collected by cardiac puncture of mice anaesthetized with ketamine (100 mg/kg) 14 days after the second immunization. Vaginal lavage fluids were obtained by gentle flushing of the mouse vagina with 100 µl of sterile PBS. Lavage fluids were then centrifuged and the supernatants were analyzed for antigen-specific antibody content. Serum, saliva, stool and vaginal lavage samples were stored at -20℃ until ELISA. Briefly, 96-well microplates (Corning, Corning, NY, USA) were coated with 50 µl of whole S. typhimurium cell lysate dissolved in PBS overnight. The plates were blocked with 1% BSA (Sigma-Aldrich) in PBS to avoid non-specific binding. A volume of 50 µl of each diluted sample was added to the plates, incubated at room temperature for 2 h, and then the plates were washed. Horseradish peroxidase conjugated goat anti-mouse IgA secondary antibody (Sigma-Aldrich), IgG1 (BD Pharmingen) or IgG2 (BD Pharmingen) was then added and incubated for 1 h at room temperature. o-Phenylenediamine dihydrochloride (0.4 mg/ml; Sigma-Aldrich) in 0.05 M phosphate citrate buffer was used as the substrate. The absorbance was measured at 490 nm in an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
The activation of COX-1 and COX-2 in Peyer's patch and ileum was observed 24 h following ginsan treatment by Western blot. Proteins were probed with monoclonal anti-COX-1, anti-COX-2, or anti-beta-actin antibody at a 1 : 1,000 or 1 : 5,000 dilution. Horseradish peroxidase-linked secondary antibodies were used at a 1:1,000 dilution. Signal detection was carried out using a LAS-3000 scanning imager (Fujifilm, Tokyo, Japan).
Expression levels of RNA transcripts were quantified using RT-PCR. Total RNA from the Peyer's patches, spleen and ileum were isolated using TRIzol reagent (Invitrogen). RNA (2 µg/tube) was reverse transcribed into cDNA for 45 min at 40℃ using Superscript RT (Promega, Madison, WI, USA) in the presence of 1 µl random primer (Promega). The cDNA was used as a template for PCR amplification (Eppendrof, Westbury, NY, USA). The reaction mixture consisted of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 200 µM dNTP (TaKaRa Bio, Shiga, Japan), 0.5 U Taq DNA polymerase (TaKaRa Bio), and 0.05 mM of each primer. The primers used were as follows: CCL3 sense, 5'-AACATCATGAAGGTCTCCAC-3', CCL3 antisense, 5'-CCAAGACTCTCAGGCATTCA-3', CCL4 sense, 5'-GTTCTCAGCACCAATGGGCTCTGA-3', CCL4 antisense, 5'-CTCTCCTGAAGTGGCTCCTCCTG-3', CCL5 sense, 5'-GGTACCATGAAGATCTCTGCA-3', CCL5 antisense 5'-AAACCCTCTATCCTAGCTCAT-3', CCL7 sense, 5'-TCTGTGCCTGCTGCTCATAG-3', CCL7 antisense, 5'-CTTTGGAGTTGGGGTTTTCA-3', CCL8 sense, 5'-CCTTCAACATGAAGATCTACGCA-3', CCL8 antisense, 5'-AACTCAGGTGTGAAGGTTCAAG-3', CCL9 sense, 5'-ATCACACATGCAACAGAGACAAAA-3', CCL9 antisense, 5'-TTATTGTTTGTAGGTCCGTGGTTG-3', CCL20 sense, 5'-TGCTCTTCCTTGCTTTGGCATGGGTA-3', CCL20 antisense, 5'-TCTGTGCAGTGATGTGCAGGTGAAGC-3', CCL21 sense, 5'-ATGGCTCAGATGATGACTCT-3', CCL21 antisense, 5'-TACTGGGCTATCCTCTTGA-3', CCL25 sense, 5'-TCACTATGAAACTGTGCCTTTTTG-3', CCL25 antisense, 5'-CTTAATTGTTGGTCTTTCTGGGC-3', COX-1 sense, 5'-AGGAGATGGCTGCTGAGTTGG-3', COX-1 antisense, 5'-AATCTGACTTTCTGAGTTGCC-3', COX-2 sense, 5'-ACACACTCTATCACTGGCACC-3', COX-2 antisense, 5'-TTCAGGGAGAAGCGTTTGC-3', β-actin sense, 5'-TCATGAAGTGTGACGTTGACATCCGT-3', and β-actin antisense, 5'-CTTAGAAGCATTTGCGGTGCACGATG-3'.
The thermal cycle conditions were 94℃ for 1 min, 55℃ for 30 s, and 72℃ for 30 s for 35 cycles. PCR products were electrophoresed on 2% agarose gels.
Splenocytes were isolated from cGFP-positive mice. Single cell suspensions were obtained by gently chopping the tissues with the needles of a 1 ml syringe and suspending the cells in RPMI 1640 containing 10% fetal bovine serum (FBS). After washing, each cell suspension was subjected to gradient centrifugation by overlaying 5 ml volumes on 5 ml Lymphoprep™ (1.077 g/ml; Nycomed Pharma, Oslo, Norway) and spinning at 400 ×g for 15 min at room temperature as recommended by the manufacturer. Interface cells were collected, washed in RPMI 1640, resuspended, and the number of viable cells was determined using the Trypan blue exclusion assay. Splenocytes (1×107) were transferred to the syngenic Balb/c mice through the caudal vein in 100µl of RPMI 1640.
Tissues were embedded in Tissue-Tek OCT compound (Miles Scientific, Naperville, IL, USA) and stored at -80℃. Cryosections 10 µm in thickness were fixed in ethanol and co-stained for phenotypic markers. Antibodies used for the phenotypic markers were as follows: CD11c (BD Pharmingen), CD3 (R&D Systems, Minneapolis, MN, USA), B220 (R&D Systems), and CCL3 (R&D Systems). All were visualized with Alexa-488 labeled or Alexa-596 labeled secondary antibody (Molecular Probes, Eugene, OR, USA). The tissues were mounted with a Slowfade™ antifade kit (Molecular Probes) and visualized using a Radiance 2100 confocal laser scanning microscope (Bio-Rad, Hercules, CA, USA).
Peyer's patches were removed from small intestines. Single cell suspensions were obtained as described earlier in the text. The cells were pooled and poured through a 100 µm nylon mesh (Millipore) prior to centrifugation for 15 min at 400 ×g. Cells were incubated on ice for 30 min in Fc block consisting of 5% mouse serum (Sigma) and 5 µg/ml anti-mouse CD32/CD16 (BD Pharmingen) in the presence of phycoerytherin-conjugated CD11c (BD Pharmingen). After staining, cells were washed and analyzed by flow cytometry with FACScan (Becton Dickson, Franklin Lakes, NJ, USA) and analyzed using CellQuest software (Becton Dickson).
Given the descriptions of ginseng extracts as being a potent vaccine adjuvant (6-8), we initially tested whether ginsan was likewise a potent adjuvant to orally administered antigens. Mice were pretreated twice with ginsan before oral immunization twice weekly for two weeks. Two weeks after the second immunization, specific antibodies against Salmonella were determined from serum, saliva, vaginal fluid and stool. Ginsan treated mice produced significantly higher amounts of serum IgG1 and IgG2 (Fig. 1A), as well as secretory IgA in all samples (Fig. 1B).
Recently, we have reported that ginsan modulates the expression of COX. Ginsan treatment suppressed allergic reaction in murine asthma model (16) and it was also effective in protecting the host from pathogenic bacterial infection via nasal route (17). Since both models were related with mucosal organs, we speculated that ginsan may also modulate COX expression in the Peyer's patch. Ginsan also specifically enhanced the mRNA expression of COX-1 and COX-2 in Peyer's patches (Fig. 2A) and it was confirmed by western blot examination (Fig. 2B).
Next, we examined various genes to elucidate the mechanism of ginsan. Among various genes, ginsan specifically increased the expression of CCL3 in the Peyer's patches, while expression was decreased both in the spleen and nearby ileum. Expression of some chemokine ligands were rather decreased following ginsan treatment (Fig. 3). Since CCL3 is related with dendritic cell (DC) migration (18,19), other chemokine ligands related with DC migration were screened. There were no changes in the expression of other genes related with DC migration in the Peyer's patches.
To determine CCL3 expressing cells within the Peyer's patches, frozen sections were stained with anti-CCL3 in combination with CD11c. CCL3 was present within the subepithelial dome region and CCL3 expression was markedly increased following ginsan treatment (Figs. 4A~D), consistent the RT-PCR results.
To determine the effect of ginsan on the DC population in the Peyer's patch, mice were sacrificed 24 h after ginsan treatment and the Peyer's patches were collected for flow cytometry analysis. Ginsan treatment significantly increased CD11c positive cells in the Peyer's patch compared to the control group (Fig. 5A). To determine if ginsan modulated DC migration, splenocytes from cGFP transgenic mice were intravenously introduced 24 h after ginsan treatment. The following day, Peyer's patches were prepared for immunofluorescence analysis of the GFP splenocyte migration. Consistent with the flow cytometry results, immunofluorescence revealed ginsan-mediated enhanced migration of GFP splenocytes to the Peyer's patches (Fig. 5B). Most of the migrated cells were found near the subepithelial dome of the Peyer's patch where DC are more prevalent (Fig. 5C). The results are consistent with the suggestion the ginsan can enhance the migration of CD11c-positive splenocytes to the Peyer's patches.
To test the role of COX on CCL3 expression regulation, mice were treated in combination with ginsan and COX inhibitors. Semi-quantitative RT-PCR for CCL3 mRNA in the Peyer's patch was determined following various treatments. The specific COX-1 inhibitor SC-560 moderately reduced the ginsan-enhanced CCL3 expression (Fig. 6A). ASA, a nonspecific COX inhibitor, mostly reduced the CCL3 expression induced by ginsan (Fig. 6B). However, single SC-560 or ASA did not totally suppress CCL3 expression suggesting that physiological CCL3 expression may be regulated by some other mechanism.
To determine the effect of ginsan and COX inhibitor on splenocyte migration, GFP splenocytes were intravenously introduced to mice pretreated with ginsan or ASA. Since COX-1 inhibition can upregulate COX-2 expression (20), ASA was used to suppress both COX-1 and COX-2. Twenty-four hours after GFP splenocyte delivery, mice were sacrificed and Peyer's patches were collected for flow cytometry analysis. Ginsan treatment increased both GFP positive and CD11c positive cells compared to the control group. ASA antagonized the effect of ginsan, reducing both CD11c cells and the migration of GFP positive cells (Fig. 7). Consistent with the CCL3 mRNA expression result, treatment with just ASA did not totally reduce the population of CD11c or the migration of DCs to the Peyer's patches.
Finally, antibody responses to oral immunization were determined. Ginsan treated mice produced significantly higher antibody titers both in stool and serum compared to that the control group. ASA suppressed antibody production induced by ginsan. Treatment just with ASA did not influence systemic antibody response, but decreased the amount of secretory IgA antibody compared to other groups (Fig. 8).
In this study, we tested if ginsan, a polysaccharide extracted from Panax ginseng, is effective in enhancing the antibody response to orally administered antigens. Ginsan significantly increased both secretory and serum antibodies to orally administered antigens suggesting that it modulates antibody response to mucosally introduced antigens.
Peyer's patches are the inductive site for the adaptive immune response to intestinal antigens (21). Foreign antigens in the gut lumen are transported to the Peyer's patches by specialized epithelial cells called M cells (22). The incoming antigens are sampled by the DCs and this sampling of antigen is believed to be critical to the induction of adaptive mucosal immunity (23). Mice defective in CCR6 lack CD11b+/CD11c+ cells in the subepithelial dome of the Peyer's patches and show an impaired humoral immune response to orally administered antigens (24). In this study, ginsan increased the population of DCs in the Peyer's patches and also enhanced the humoral immune response to orally administered Salmonella antigen. However, CCR6 expression was not influenced by ginsan treatment.
We have previously reported that ginsan modulates COX expression in the lung and nasal associated lymphoid tissues. In murine asthma model, ginsan suppressed the allergic reaction by up-regulating COX expression that is down-regulated upon allergen challenge (16). Likewise, when V. vulnificus was nasally challenged, it suppresses COX expression and ginsan maintained the COX expression (17). In both model, ginsan modulated the expression of COX at the effector site which is the mucosal system.
Therefore, we hypothesized that ginsan may also have influenced the COX expression in the Peyer's patch. The expression of COX was distinctively increased in the Peyer's patch of ginsan treated mice. In a septic mouse model, ginsan significantly decreases the proinflammatory cytokines including tumor necrosis factor-alpha, interleukin (IL)-1β, IL-6, IL-12, IL-18 and interferon-alpha (10), strongly supporting that ginsan does not induce pathologic inflammatory response in the recipient.
To understand the relation between COX expression enhanced antibody response to orally administered antigen, various genes related with cell migration, B cell or T cell responses were screened. Among various genes, the expression of CCL3 was distinctively increased in the Peyer's patch following ginsan treatment. CCL3 is a potent chemoattractant for monocytes (25), natural killer cells (26), eosinophils (27) and DCs (18,19). When CCL3 is administered to mice, it induces mobilization of DC precursors into the circulation (28). Furthermore, when CCL3 was nasally inoculated with ovalbumin, higher antibody titers of IgG and IgA are detected suggesting CCL3 can enhance both systemic adaptive and mucosal immunity (29). The enhanced expression of CCL3 at the Peyer's patches induced by ginsan may be responsible for the migration of DCs; alternatively, CCL3 expressing DCs could have migrated to the subepithelial dome of the Peyer's patches.
Expression of other chemokines were either down-regulated or unchanged. Ginsan treatment decreased the expression of CCL5 in the spleen and ileum and CCL8 in the Peyer's patch and ileum. Also, expression of CCL21 and CCL25 were also decreased in the ileum in the ginsan treated group. This suggests that the increased expression of the CCL3 in the Peyer's patch is not likely due to a pathologic inflammatory response involving recruitment of lymphocytes to the inflammatory focus (30).
To test the role of COX in modulating CCL3, COX was specifically inhibited by inhibited by SC-560 or nonspecifically inhibited by ASA. Both treatments reverted the expression of CCL3 to the control level. However, additional suppression was not observed suggesting that the regulation of the constitutive expression of CCL3 may differ from that induced by ginsan. The migration of GFP splenocytes was also reversed by the COX inhibitors, as was both the systemic and mucosal immune responses enhanced by ginsan. Treatment solely with COX inhibitor impaired only the mucosal immune response compared to the normal systemic immune response. In conclusion, ginsan enhances antibody response to orally introduced antigens by modulating the COX expression in the Peyer's patches. Enhanced COX expression increases DC migration to the Peyer's patch via CCL3. Since extracts from P. ginseng are wide used in the oriental medicine, these findings suggest that ginsan may serve as an efficient supplement for the oral vaccine.
Figures and Tables
 | Figure 1Ginsan induces higher humoral immune response against paraformaldehyde fixed S. typhimurium. BALB/c mice immunized with 1×109 colony forming units fixed Salmonella twice each week for two weeks. Ginsan was applied one day before oral immunization. Two weeks after the final immunization, the mice were sacrificed and saliva, vaginal wash, stool and serum were obtained. (A) Salmonella specific IgG in serum. (B) Salmonella specific IgA in saliva, vaginal flush or stool. Each group consists of five mice (*p<0.05). |
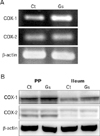 | Figure 2Effect of ginsan on COX expression in Peyer's patches. (A) Semi-quantitative RT-PCR for COX-1 and COX-2 mRNA from the Peyer's patches. Total RNA was isolated and used for RT-PCR. PCR products were visualized on 2% agarose gel using ethidium bromide. (B) Western blotting of COX-1 and COX-2 in the Peyer's patch and ileum. Increased COX-1 expression was evident using both approaches. |
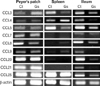 | Figure 3Effect of ginsan on various chemokine mRNA expression. Semiquantitative RT-PCR analysis of CCL expression in the Peyer's patch, spleen and ileum were analyzed. Total RNA isolated following ginsan treatment was used for RT-PCR. PCR products were visualized on 2% agarose gel using ethidium bromide. Ginsan specifically enhanced CCL3 expression in the Peyer's patch. |
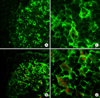 | Figure 4Localization of CCL3 in the Peyer's patches. Frozen sections of Peyer's patches were doubly stained with antibodies against CD11c (green) in combination with anti-CCL3 (red). Panels (A) and (C) display the controls, and panels (B) and (D) display the ginsan treatment. (A, B ×600; C, D ×1,800). |
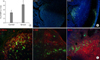 | Figure 5Analysis of the migration of splenocytes to the Peyer's patches. (A) Ginsan treatment significantly enhanced CD11c positive cell numbrs in the Peyer's patch. (*p<0.05) (B) BALB/c mice were pretreated with ginsan for two days. GFP splenocytes (5×106) were then intraveneously introduced. To determine migration of the splenocytes, Peyer's patches were fixed and examined. Ginsan treatment greatly enhanced migration of GFP splenocytes to the subepithelial dome of Peyer's patches. (C) Newly migrating cells were mostly found near CD11c positive cells. |
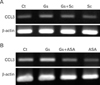 | Figure 6Effect of ginsan and COX-1 specific inhibitor (A) or non-specific COX-inhibitor (B) on the expression of CCL3 mRNA in Peyer's patches. Total RNA was isolated and used RT-PCR can was prepared by reverse transcription. The PCR products were visualized on 2% agarose gel using ethidium bromide. CCL3 expression was antagonized by SC-560 and aspirin (ASA) treatment. (Ct, PBS; Gs, Ginsan; Sc, SC-560; ASA). |
 | Figure 7Effect of ginsan and COX inhibitor on cell migration to the Peyer's patches. Cells from Peyer's patches were prepared following various treatments and GFP splenocyte transfer. Cells were stained with antibodies against CD11c. CD11c cells were increased following ginsan treatment, which was antagonized by ASA treatment (*p<0.05). |
ACKNOWLEDGEMENTS
This work was supported by grant No. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE).
References
2. Brokstad KA, Eriksson J, Cox RJ, Tynning T, Olofsson J, Jonsson R, Davidsson A. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002. 185:878–884.

3. Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000. 13:523–533.

4. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002. 60:258–274.

5. Lu J, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009. 7:293–302.

6. Rivera E, Hu S, Concha C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine. 2003. 21:1149–1157.

7. Hu S, Concha C, Lin F, Persson Waller K. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet Immunol Immunopathol. 2003. 91:29–37.

8. Rivera E, Daggfeldt A, Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. 2003. 91:19–27.

9. Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold [corrected]. Drugs Exp Clin Res. 1996. 22:65–72.
10. Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006. 36:37–45.

11. Han Y, Son SJ, Akhalaia M, Platonov A, Son HJ, Lee KH, Yun YS, Song JY. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid Based Complement Alternat Med. 2005. 2:529–536.

12. Ivanova T, Han Y, Son HJ, Yun YS, Song JY. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem Toxicol. 2006. 44:517–521.

13. Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, Yun YS. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998. 64:110–115.

14. Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002. 24:469–482.

15. Song JY, Han SK, Son EH, Pyo SN, Yun YS, Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002. 2:857–865.

16. Lim YJ, Na HS, Yun YS, Choi IS, Oh JS, Rhee JH, Cho BH, Lee HC. Suppressive effects of ginsan on the development of allergic reaction in murine asthmatic model. Int Arch Allergy Immunol. 2009. 150:32–42.

17. Na HS, Lim YJ, Yun YS, Choi YH, Oh JS, Rhee JH, Lee HC. Protective effect of Ginsan against Vibrio vulnificus infection. J Bacteriol Virol. 2009. 39:113–118.

18. Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995. 155:3292–3295.
19. Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, D'Amico G, Power CA, Wells TN, Gobbi M, Allavena P, Mantovani A. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997. 159:1993–2000.
20. Tanaka A, Hase S, Miyazawa T, Takeuchi K. Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J Pharmacol Exp Ther. 2002. 300:754–761.

21. Rimoldi M, Rescigno M. Uptake and presentation of orally administered antigens. Vaccine. 2005. 23:1793–1796.

22. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000. 16:301–332.

23. Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, Nilsen EM, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999. 20:141–151.

24. Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000. 12:495–503.

25. Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995. 63:3871–3877.

26. Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995. 155:3877–3888.
27. Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992. 176:1489–1495.

28. Zhang Y, Yoneyama H, Wang Y, Ishikawa S, Hashimoto S, Gao JL, Murphy P, Matsushima K. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004. 96:201–209.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download