Abstract
Background
Monoclonal antibodies (mAbs) recognizing Class III epitope of CD34 are essential for flow cytometric diagnosis of leukemia.
Methods
27H2 mAb was developed from a mouse alternatively immunized with human acute leukemia cell lines, KG1 and Molm-1. Using flow cytometric analysis of various leukemic cell lines and peripheral blood, immunohistochemical study of frozen tonsil, we characterized 27H2 mAb. Antigen immunoprecipitated with 27H2 mAb immunobloted with anti-CD34 mAb. A case of bone marrow sample of acute lymphoblastic leukemia (ALL) patient was obtained at CBNU Hospital. For epitope identification enzyme treatment with neuraminidase and O-sialoglycoprotein endopeptidase (OSGE) and blocking assay with known classIII mAb (HPCA-2) were done.
Results
Only KG1 and Molm-1 revealed positive immunoreactivity. Immunohistochemical staining disclosed strong membranous immunoreactivity on high endothelial venules. Antigen immunoprecipitated by 27H2 mAb showed approximately 100 kDa sized band immunoblotted with anti-CD34 under non-reducing conditions. Epitope recognized by 27H2 mAb disclosed resistancy to both neuraminidase and OSGE treatment and completely blocked with known class III mAb preincubation. CD34 positive leukemic cells in BM of pre B cell ALL patient detected by FITC-conjugated 27H2 and HPCA-2 were identified with similar sensitivity.
The CD34 is a heavily glycosylated transmembrane protein, which was expressed on developmentally early hematopoietic stem and progenitor cells (1,2) and small-vessel endothelial cells (3-5), especially high endothelial venules on lymph nodes. CD34 is used as a marker for leukemia diagnosis and subclassification, as a label for quantification of stem/progenitor cells in blood and marrow and as a target for immunologic purification of stem/progenitor cells for clinical transplantation (6). Until recently lots of mAbs against CD34, which were mainly established by immunizing leukemic cell lines has provided essential roles for structural and functional analyses of the CD34 antigen.
The 5th HLDA workshop approved the three epitopes of CD34 on the basis of differential sensitivity of the three epitopes to the actions of neurminidase (sialidase from Vibrio Cholera) and glycoprotease (or endopeptidase from Pasteurella haemolytica) as a standard for the classification of new CD34 antibodies. The class I epitope is sensitive to all proteolytic enzymes (glycoprotease, neuraminidase and chymopapain). Early established mAbs (My10, BI3C5, 12.8) obtained by immunizing relatively undifferentiated leukemic cells are included in this category and recognize heavily O-glycosylated epitopes. The class III epitope is insensitive to all proteolytic enzymes. HPCA-2 (also known as 8G12) mAb avidly bind to all glyforms of CD34 (7-10).
The class II epitope is resistant to neuraminidase, but sensitive glycoprotease. As QBEnd 10 class II mAb recognize denaturation resistant epitope, it is generally used in immunopathological diagnosis using paraffin embedded tissues.
Because leukemia arise from immature hematopoietic precursor cells during differentiation, CD34 is one of leukemia markers for differential diagnosis. About 40% of acute myeloid leukemias and 65% of pre-B acute lymphoblastic leukemias express the CD34 molecule, whereas only 1~5% of acute T-lymphoid leukemias express the CD34 antigen. CD34 is often expressed on blasts from chronic myeloid leukemia patients in blast crisis, whereas chronic phase cells, other chronic leukemias and lymphomas of more differentiated phenotypes are uniformly negative (1,2,11-17). For the flow cytometric detection of leukemic cells in leukemia patient class III mAbs are known to be better than class I and II mAbs (7,8,18-26). It was reported that at least some class I antibodies fail to detect the glycoforms of CD34 expressed some leukemias and leukemic cell lines because lineage committed progenitors lose their carbohydrate moieties. Furthermore if they are conjugated with negatively charged fluorochromes such as FITC their identification rates of leukemic cells in patient are markedly reduced below the threshold of practical usage. On the contrary unconjugated class III mAbs avidly bind to all glycoslated backbone of CD34 (7-10) and after conjugation with appropriate fluorochromes relatively hold their high specificity and avidity for all glycosylated structures of CD34. So it seems to be that class III mAbs are the best reagents of identification of both leukemic cells and normal BM stem cells by flow cytometric analysis.
In this study, we produced a high affinity monoclonal antibody (27H2) to CD34 using a new alternative immunization strategy and confirmed class III epitope specificity. And we also revealed a diagnostic usefulness of FITC conjugated 27H2 mAb in pre B cell acute leukemia patient.
Balb/c mice, 6~8 weeks old, of either sex, were purchased from Daihan animal Corporation (seoul, south Korea). Normal peripheral blood in ethylenediaminetetraacetic acid tubes was obtained from healthy volunteers. The leukemic cell lines, KG-1, Molm-1, Jurkat, CEM, Daudi, TF-1, IM9 and Ramos were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Bone marrow cells of acute myeloid leukemia patient and Human frozen tissue were collected from the Department of Pathology, Chungbuk National University Hospital. Anti-CD34 (clone 27H2 and 4H11), anti-CD3, anti-CD8, anti-CD19, anti-CD11b, anti-CD66c, anti-CD61 were either purchased from DiNona Inc. (clone 27H2 and 4H11, South Korea). Other anti-CD34 and CD59 were either purchased from BD Biosciences (clone QBEnd 10 and clone HPCA-2). and anti-CD120a was either purchased from Department of Pathology, Chungbuk National University.
After 6 week-old Balb/c mouse were immunized intraperitoneal (I.P.) injection with Molm-1 and KG1 cells per mice at 4-2-2 week intervals for 2 month. The unique method of immunization was alternatively performed by Molm-1 and KG1 leukemic cell line. First Molm-1 cells were injected on the first day. 30 days later, Molm-1 cells were injected. Third immunized and final boost were injected KG-1 cells. Test bleed was performed in Molm-1 and KG1 by FACS. The hybrids by fusion the B cells and myeloma using PEG were selected in HAT medium. After 10 days, culture supernatants were harvested and tested for reaction to human lymphocytes by indirect immunofluorescence method using flow cytometry. The resulting hybridoma clone, whose supernatant was reactive to human lymhpocytes, was named 27H2. Cells from 96 well were subcloned by limiting dilution, and the culture supernatants of the clones were tested for antibody production. Finally, we established a hybridoma clone, which is producing 27H2 monoclonal antibody (DiNonA Inc. Korea).
Tonsil and thymus frozen tissue sections were obtained from the surgical pathology files of Chungbuk National University Hospital and screened. Tissues were incubated with 27H2 mAb obtained from hybridoma supernatant at 4℃ for overnight, thereafter, were washed four times with TBS. Sequential incubation was done with biotinylated anti-mouse immunoglobulin (DAKO, Carpinteria, USA) for 10 min at room temperature (RT). Then tissues were washed four times again and followed streptavidin-horseradish peroxidase (DAKO, Carpinteria, USA) for 10 min at RT, were washed the same. Finally, reaction product was visualized with 3'-diaminobenzidine (DAB; Sigma, USA). Counterstain was not performed and the reaction pattern was analyzed based on serial hematoxylin-eosin stained sections. Positive cells were defined by the positive staining pattern along the cell membrane.
1.25×107 cells/ml of TF-1 cells and 1.25×108 cells/ml of bone marrow cells of acute myeloid leukemia patient were lysed with 1 ml of ice-cold lysis buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40; NP40 and 1 mM phenyl-methyl-sulfonyl-fluoride; PMSF). For searching of 27H2 mAb recognized antigen, lysates were incubated with 27H2 mAb and isotype matched control anti-CD120a coated protein G sepharose bead for 16~18 hr at 4℃. After extensive washing, the immunoprecipitates were eluted by boiling for 10 min with 5×sample buffer and analyzed on 10% SDS-PAGE under both non-reduced and reduced conditions. After eletrophoretic transfer of the proteins to a PVDF (Millipore Corporation, USA), the membrane was blocked with 5% skim milk in 1×PBS containing 0.02% Tween-20. The membrane was incubated with anti-CD34 mAb (4H11), followed by peroxidase-conjugated goat anti-mouse Ig (Dako, USA, 1:1,000 dilution in blocking solution). Immunoreactive proteins were visualized using the enhanced chemiluminescence detection system (ECL, iNtRON).
To identify the epitope of CD34 mAb, TF-1 cells at 3.5×106/ml in Ca++/Mg+ free HBSS (Hank's Balanced Salt Solution)cells were harvested and washed by 1×PBS two times. Neuraminidase (0.08 U) and O-sialoglycoprotein endopeptidase (10 ul) was added to 200 ul total volume. Then, cells were incubated for 30 minutes at 37℃, washed with cold 1×PBS and stained with 27H2, K06 and QBEnd 10, respectively. Cells washed again and then incubated with FITC conjugated secondary antibody and analyzed by flow cytometry (FACSCalibur, BD, USA). K06 and QBEnd 10 were used as positive control mAbs, respectively.
KG-1 and Molm-1 cells were preincubated with 1, 10 and 20 µg/ml of purified HPCA-2 (BD Bioscience) mAbs, respectively, for 30 min at 4℃. Then, cells were stained with 1µg/ml 27H2-FITC (DiNonA Inc. Korea), incubated for 15 min at 4℃, where samples blocked off the light, washed with ice-cold 1×PBS, analyzed using FACSCalibur (Becton-Dickinson, San Jose, CA).
Screening of cell surface antigen was recognized 27H2 (DiNonA Inc. Korea) and HPCA-2 mAb (BD Bioscience) was analysis by immunofluorescence staining and flow cytometeric analysis. Lymphocytes, monocytes, granulocytes, platelets and RBCs which were isolated from peripheral blood of healthy volunteer were obtained and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The indirect immunofluorescence staining was performed by sequentially incubating 106 cells with saturating amounts of culture supernatant or 1µg of purified antibody of 27H2 mAb or 2.0µg of purified antibody of HPCA-2 mAb, for 20 min at 4℃. Thereafter, cells were washed two times with cold phosphate-buffered saline (PBS) followed by FITC- or PE-conjugated goat anti-mouse IgG (CALTAG, USA) for 15 min at 4℃ then cells were washed two times with cold phosphate-buffered saline (PBS). Cells were analyzed with a flow cytometer (FACScalibur, BD). The double color staining was performed by sequentially incubating 106 cells with saturating amounts of culture supernatant or 10µg of FITC conjugated antibody of 27H2 and HPCA-2, PE conjugated antibody of HLA-DR (YG18; DiNona Inc, Korea) mAb, for 20 min at 4℃. Cells were analyzed with a flow cytometer (FACScalibur, BD).
The isotype of 27H2 mAb was determined by enzyme immunoassay using mouse immunoglobulin isotyping kit. The isotype of 27H2 mAb was IgG1.
To examine expression profiles of antigen recognized by 27H2 mAb, we performed flow cytometric analysis against normal peripheral blood cells and various kinds of leukemic cell lines. Only two KG1 and MOLM1 leukemic cells showed strong immunoreactivity with 27H2 mAb, while other Jurkat, Molt4, U937, IM9, Ramos, CEM, and Daudi leukemic cells did not reveal any immunoreactivity (Table I). None of the mature hematopoietic cells including lymphocytes, granulocytes, platelet and red blood cells were reacted with 27H2 mAb as expected (Table I).
To evaluate tissue distribution patterns of antigen recognized by 27H2 mAb we did immunohistochemical staining. The endothelial cells of small vessels of all frozen tissues tested revealed moderate positive immunoreactivity. Especially, 27H2 mAb was strongly reacted on high endothelial venules throughout the tonsil (Fig. 1). But all lymphocytes, including mature T lymphocytes in interfollicular area, resting B lymphocytes in mantle zone, activated B lymphocytes in germinal center, and squamous epithelia on tonsil showed negative immunoreactivity (Fig. 1). 27H2 mAb disclosed no immunoreactivity on paraffin embedded tonsil tissue section (data not shown).
To characterize antigen recognized by 27H2 mAb, we performed immunoprecipitation in TF-1 cells and bone marrow samples of acute myeloid leukemia patient using 27H2 mAb and subsequently did western blot analysis by known CD34 mAb (4H11, Dinona, Inc). As shown in Fig. 2, Western blot disclose discrete band of an approximate molecular weight of 100 kDa as expected in lane 1 and 3 under non-reducing condition (Fig. 2). So we could show direct evidence that 27H2 mAb can recognize CD34 antigen.
To identify the epitope specificity we treated TF-1 cells with two kinds of enzymes. Upon treatment with neuraminidase, which can cleave sialylated carbohydrate moiety and O-sialoglycoprotein endopeptidase, which can cleave sialylated 0-linked glycans residue, both K06 mAb as a positive control showed sensitive epitope to neuraminidase (Fig. 3A) and QBEnd 10 class II mAb revealed sensitve epitope to endopeptidase (Fig. 3B). However epitope recognized by 27H2 mAb was resistant both neuraminidase and O-sialoglycoprotein endopeptidase treatment in TF-1 leukemic cells (Fig. 3). This results tell us that 27H2 mAb recognize class III epitope of CD34.
Whether epitope recognized 27H2 mAb is blocked by well known mAb (Anti-HPCA-2, BD) against CD34 class III epitope, we performed blocking assay. Pre incubation with anti-HPCA-2 near completely blocked classIII epitopes in dose dependent manner, so FITC conjugated 27H2 mAb could not bind at all on TF-1 cells under high concentration (Fig. 4). This dose dependent inhibition means that two anti-HPCA-2 and 27H2 mAb recognize an identical epitope or at least shared epitopes. Therefore, we also confirmed that the antibody 27H2 recognize class III epitope of CD34.
To evaluate the diagnostic usuage of this 27H2 mAb we examined the detection sensitivity of class III epitope on leukemic cells of pre B ALL patient using FITC conjugated commercial mAbs, anti-HPCA-2, and FITC conjugated 27H2 mAb by flow cytometric analysis. We confirmed that both 27H2 and Anti-HPCA-2 epitopes revealed similar detection sensitivity and affinity on pre B ALL (Fig. 5). The average percentage of detecting CD34 positive leukemic cells in bone marrow sample using 27H2 and anti-HPCA-2 mAb was equivalent to 95%.
In this report we produced a high affinity monoclonal antibody (27H2) to CD34 using a new alternative immunization strategy and confirmed that 27H2 recognized class III epitope of CD34 showing resistancy to neuraminidase and endopeptidase treatment and inhibition with well known CD34 class III mAb (HPCA-2).
Complexity of glycoslyation along differential stage of hematopoietic progenitor cells and differently glycosylated status of leukemic cells can generate many conformation dependent novel epitopes of CD34. So, new mAbs recognizing novel different epitopes is still needed for further clarification of CD34 structure and function. In order to establish more appropriate mAb against CD34 in flow cytometric diagnosis of leukemia, we planed alternative cellular immunization method. On the hypothesis that if we alternatively immunize KG1 and Molm1, which express high level of CD34, chance to obtain mAbs recognizing more common conformation dependent epitopes of CD34 (probably class III epitope) would be increased. We screened and picked up hybridoma clones which are producing mAbs which reveal positive immunoreactivity to both KG1 and Molm1 but negative normal PBMC. Among several clones selected 27H2 showed most high mean fluorescent intensity in flow cytometric analysis. In addition, 27H2 mAb was not reacted with platelets, monocytes, granulocytes, lymphocytes, and erythrocytes as expected (data not shown). In the case of various leukemic cell lines, only KG1 (acute myelogenous leukemia) and Moml-1 (megakaryoblast) revealed strong immunoreactivity with 27H2, but other T and B leukemic cell lines were not reacted with 27H2 (Table I). Sensitivity and specificity studies to detect CD34 positive leukemic cells compared with commercial CD34 mAb (HPCA-2) were achieved (Table I).
Immunoreactivity on frozen tonsil section by 27H2 mAb disclosed strong positivity on high endothelial venules as expected, while all mature and actvated lymphocytes and squamous epithelia revealed negative immunoreactivity (Fig. 1). Due to narrow tissue distribution patterns of CD34 antigen, only positive on small blood vessels in tissue section, this immunohistochemical staing results and above negative normal blood cell expression patterns strongly support the possibility that 27H2 mAb can recognize CD34. Furthermore 27H2 mAb failed to detect formalin fixed paraffin embedded CD34 antigen. As only mAb (eg. QBEnd 10) recognizing CD34 class II epitope can detect paraffin embedded CD34, these finding indirectly support 27H2mAb can recognize CD34 class I or class III epitope other than classII.
Nextly by perfoming immunoprecipitation with 27H2 mAb and subsequently did Western blotting by known CD34 mAb, we could disclose direct evidence that antigen recognized by 27H2 mAb seems to be CD34 (Fig. 2).
Sutherland et al. (27) reviewed structural and functional features of the CD34 antigen and definitively showed academical and clinical importance of different mAbs against CD34. Because epitope specificity of mAbs against CD34 determines their specific clinical and research usage, identification of specific epitope is inevitable step. Standard of epitope classification was adopted by the 5th HLDA workshop. MAbs to epitopes sensitive to both sialidase and glycopeptidase designated class I (BI.3C5) and mAbs resistant to both enzyme designated class III (HPCA-2). MAbs (QBEnd 10) against epitope sensitive to glycopeptidase, but resistant to neuraminidase defined class II. In this study the epitope recognized by 27H2 was resistant by neuraminidase and endopeptidase treatment (Fig. 3) and completely blocked with known classIII mAb (HPCA-2) (Fig. 4), so it was evident that 27H2 recognize class III epitope of CD34.
For the fluocytometric detection of leukemic cells in leukemia patient class III mAbs are known to be better than class I and II mAbs (7,8,18-26). At least three factors involved are firstly charge of the epitope, secondly avidity between epitope and mAbs and lastly glycosylated status of epitope. It was reported that at least some class I antibodies fail to detect the glycoforms of CD34 expressed some leukemias and leukemic cell lines because lineage committed progenitors lose their carbohydrate moieties. Furthermore, if they are conjugated with negatively charged fluorochrome such as FITC and their identification rates of leukemic cells in patient are markedly reduced below the threshold of practical usage. Class II mAb-FITC conjugates no longer bind with high affinity due to charge constraints (7,23). On the contrary unconjugated class III mAbs avidly bind to all glycosylated backbone of CD34 (7-10) and after conjugation with appropriate fluorochromes relatively hold their high specificity and avidity for all glycosylated structures of CD34. It was also reported that class III (HPCA-2)-FITC detected a large number of CD34 positive leukemic cells in normal marrow and acute leukemia samples than class II (QBEnd 10)-FITC conjugates (7,8,18-22). So it seems to be widely accepted that CD34 class III mAbs are the best reagents of identification of both leukemic cells and normal BM stem cells by flow cytometric analysis. As FITC conjugated 27H2 disclosed similar sensitivity (95%) and affinity (Fig. 5) compared with widely used commercial class III (HPCA-2)-FITC in pre B cell acute leukemia patient, 27H2 class III mAb can be clinically used as a useful diagnostic reagent.
Figures and Tables
 | Figure 1Immunoreactivity of 27H2 on frozen tonsil. Frozen tonsil sections were immunohistochemically stained with monoclonal antibody 27H2 and counter-stained with hematoxylin. 27H2mAb show strong immunoreactivity on endothelial cells of high endothelial venules (Arrow, A), but mature lymphocytes (A) and squamous epithelia (Arrow head, B) reveal negative immunoreactivity (A: ×200, B: ×40). |
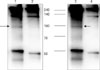 | Figure 2Conformation of antigen recognized by 27H2 mAb as CD34. For searching of 27H2 mAb recognized antigen, TF-1 cells (lane 1, 2) and Bone marrow cells of acute myeloid leukemia patient (lane 3, 4) were lysed. Lysates were incubated with 27H2 mAb (lane 1, 3) and isotype matched control anti-CD120a (2, 4) coated protein G sepharose bead. The immunoprecipitates were separated by SDS-PAGE and electro-blotted with 5% skim milk. Membrane was incubated with anti-CD34 mAb (4H11), followed by peroxidase-conjugated goat anti-mouse Ig. Immunoreactive proteins were visualized using the enhanced chemiluminescence detection system. Note discreate band of about 100 kDa sized as expected in lane 1 and 3 (Arrows). |
 | Figure 3Identification of class III epitopes of CD34 by neuraminidase and endopeptidase treatment in TF-1 cells. (A) Identification of epitope using neuraminidase enzyme treatment. 27H2 showed neuraminidase resistant class II or III epitope, while. K06 revealed neuraminidase sensitive epitope. (B) Identification of epitope using O-sialoglycoprotein endopeptidase enzyme treatment. 27H2 showed endopeptidase resistant class III epitope, while. QBEnd 10 revealed endopeptidase sensitive class II epitope. |
 | Figure 4Identification of class III epitopes of CD34 by blocking assay with class III anti-HPCA-2 in TF-1 cells. Blocking assay with anti-HPCA-2 and 27H2. TF-1 cells were preincubated with various amounts of anti-HPCA-2 and then FITC conjugated 27H2 was incubated as detection antibody. Note competitive inhibition of class III anti-HPCA was definitive. The data are representative of three separate experiments performed. |
 | Figure 5Comparison with FITC conjugated commercial class III CD34 mAb (Anti-HPCA-2) in bone marrow cells of pre B cell acute lymphoblastic leukemia patient. Shown are the determinations of leukemic cells from a patient with pre B cell acute lymphoblastic leukemia. Staining was done with IgG FITC, PE, 27H2-FITC, Anti-HPCA-2-FITC, 4H11-FITC and HLA-DR (YG18)-PE. Note similar sensitivity for detecting leukemic cells of pre-B cell acute lymphoblastic leukemia patient. |
ACKNOWLEDGEMENTS
This work was supported in part by a research grant (no. DN-05-02 and DN-06-01) from the DiNonA R&D Project, Iksan, Korea.
References
1. Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984. 133:157–165.
2. Tindle RW, Nichols RA, Chan L, Campana D, Catovsky D, Birnie GD. A novel monoclonal antibody BI-3C5 recognises myeloblasts and non-B non-T lymphoblasts in acute leukaemias and CGL blast crises, and reacts with immature cells in normal bone marrow. Leuk Res. 1985. 9:1–9.

3. Watt SM, Karhi K, Gatter K, Furley AJ, Katz FE, Healy LE, Altass LJ, Bradley NJ, Sutherland DR, Levinsky R, et al. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells. Leukemia. 1987. 1:417–426.
4. Beschorner WE, Civin CI, Strauss LC. Localization of hematopoietic progenitor cells in tissue with the anti-My-10 monoclonal antibody. Am J Pathol. 1985. 119:1–4.
5. Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990. 75:2417–2426.

6. Civin CI, Small D. Purification and expansion of human hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 1995. 770:91–98.

7. Greaves MF, Brown J, Molgaard HV, Spurr NK, Robertson D, Delia D, Sutherland DR. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992. 6:Suppl 1. 31–36.
8. Sutherland DR, Keating A. The CD34 antigen: structure, biology, and potential clinical applications. J Hematother. 1992. 1:115–129.

9. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996. 5:213–226.
10. Siena S, Bregni M, Brando B, Belli N, Lansdorp PM, Bonadonna G, Gianni M. Flow cytometry to estimate circulating hematopoietic progenitors for autologous transplantation: comparative analysis of different CD34 monoclonal antibodies. Haematologica. 1991. 76:330–333.
11. Katz FE, Tindle R, Sutherland DR, Greaves MF. Identification of a membrane glycoprotein associated with haemopoietic progenitor cells. Leuk Res. 1985. 9:191–198.

12. Shpall EJ, Jones RB, Franklin W. Transplantation of autologous CD34+ hematopoietic progenitor cells into breast cancer patients following high-dose chemotherapy. J Clin Oncol. 1994. 12:28–32.
13. Vescio R, Schiller G, Stewart AK, Ballester O, Noga S, Rugo H, Freytes C, Stadtmauer E, Tarantolo S, Sahebi F, Stiff P, Meharchard J, Schlossman R, Brown R, Tully H, Benyunes M, Jacobs C, Berenson R, DiPersio J, Anderson K, Berenson J. Multicenter phase III trial to evaluate CD34(+) selected versus unselected autologous peripheral blood progenitor cell transplantation in multiple myeloma. Blood. 1999. 93:1858–1868.
14. Civin CI, Trischmann TM, Fackler MJ, Bernstein ID, Bühring HJ, Campos L, et al. In : Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, editors. M7.1. Report on the CD34 cluster workshop. Leukocyte typing IV. White cell differentiation antigens. 1989. Proceedings of the 4th International Workshop and Conference; 1989 Feb 21-25; Vienna, Austria. New York, Tokyo: Oxford University Press;818–825.
15. Greaves MF, Titley I, Colman SM, Buhring H-J, Campos L, Castoldi GL, et al. Schlossman S, editor. Report on the CD34 cluster Workshop. Leukocyte Typing V. 1995. Oxford University Press;840–846.
16. Nishio H, Tada J, Hashiyama M, Hirn J, Ingles-Esteve J, Suda T. In : Kishimoto T, Kikutani H, von dem Borne AEG, Goyert SM, Mason DY, Miyasaka M, editors. MC7. CD34 workshop panel report. Leucocyte typing VI. White cell differentiation antigens. 1997. Proceedings of the 6th International Workshop and Conference; 1996 Nov 10-14; Kobe, Japan. New York, London: Garland Publishing Inc.;974–984.
17. Titley I, Healy LE, Scott M, Amos TA, Gordon MY. Extent of variability inherent in measurements of CD34-positive cells in different human haemopoietic tissues. Bone Marrow Transplant. 1995. 16:611–616.
18. Titley I, Healy LE, Scott M, Amos TA, Gordon MY. Extent of variability inherent in measurements of CD34-positive cells in different human haemopoietic tissues. Bone Marrow Transplant. 1995. 16:611–616.
19. Cannovo N, Bossy D, Hirn J. Significant diversity of the reactivity of monoclonal CD34 antibodies requires an extended classification of the CD34 epitopes. Lab Hematol. 1997. 3:253–260.
20. Serke S, Huhn D. Expression of class I, II and III epitopes of the CD34 antigen by normal and leukemic hemopoietic cells. Cytometry. 1996. 26:154–160.

21. Steen R, Tjønnfjord GE, Gaudernack G, Brinch L, Egeland T. Differences in the distribution of CD34 epitopes on normal haemopoietic progenitor cells and leukaemic blast cells. Br J Haematol. 1996. 94:597–605.

22. Lanza F, Moretti S, Castagnari B, Latorraca A, Rigolin GM, Bardi A, Castoldi G. CD34+ leukemic cells assessed by different CD34 monoclonal antibodies. Leuk Lymphoma. 1995. 18:Suppl 1. 25–30.

23. Weinberg DS, Benjamin RJ. QBEnd10 (CD34) antibody is unsuitable for routine use in the ISHAGE CD34+ cell determination assay. J Hematother. 1996. 5:599–603.
24. Macey MG, McCarthy DA, vanAgthoven A, Newland AC. How should CD34+ cells be analysed? A study of three classes of antibody and five leucocyte preparation procedures. J Immunol Methods. 1997. 204:175–188.

25. Sovalat H, Racadot E, Hénon P, Fuchs P, Lewandowski H, Billot M. Comparative analysis of class I, II and III epitope-detecting CD34 monoclonal antibodies by quantitative flow cytometry. Hematol Cell Ther. 1998. 40:259–268.
26. Sutherland DR, Anderson L, Nayar R, Keeney M, Chin Yee I. Ball E, Lister J, editors. The ISHAGE Guidelines for CD34+ cell determination: Applications in autologous and allogenic blood and marrow transplantation. Bone Marrow Transplantation: A Practical Approach. 2000. Churchill Livingstone;239–246.
27. Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents. 2001. 15:1–13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download