INTRODUCTION
Kalopanax pictus (KP) NAKAI (Araliaceae) is distributed in Korea, Japan, and China. The stem bark of KP has been used in traditional medicine to treat rheumatoidal arthritis, neurotic pain and diabetes mellitus (1). The constituents such as saponins, polyacetylenes, phenylpropanoid glycosides, lignans, and simple phenolic glycosides have been isolated from this plant (2). Kalopanaxsaponin A, the constituent of KP, has been reported to inhibit iNOS, COX-2 expression, and TNF-alpha release in vitro (1). However, the underlying mechanism of KP function has remained to be characterized so far.
Nitric oxide (NO) is a free radical with multiple effects on various organ systems. The most prominent physiological actions of NO as a biological mediator include cGMP-dependent vasodilation, neural communication, host defense, inflammation, immune suppression and blood clotting (3). NO is produced in physiological and pathophysiological conditions by NO synthase (NOS), and inducible NOS (iNOS) is induced by inflammatory cytokines and/or bacterial lipopolysaccharide (LPS) in various cell types including macrophages. A large amount of NO, particularly synthesized by iNOS, induces an inflammatory response to inhibit the growth of invading microorganisms and tumor cells. This strong inflammatory response to foreign cells could also cause further damage for the neighboring cells and tissues of the host. Therefore isozyme specific inhibitors of NOS are essential for therapeutic purposes and drugs that specifically inhibit iNOS could be useful in treating diseases mediated by NO overproduction (4). A major transcription factor that regulates iNOS gene expression is NF-κB. In unstimulated cells, NF-κB is present in the cytosol bound to the inhibitory protein I kappa B (IκB). In response to stimulation such as LPS, IκBs are rapidly ubiquitinated and degraded by 26S proteasome complex. The free NF-κB dimers translocate to the nucleus and stimulate target genes expression. The critical event which triggers the degradation of IκBs is their stimulus-dependent phosphorylation at two serine residues (Ser32 and 36) that are located within their conserved N-terminal regulatory region.
Heme oxygenase-1 (HO-1) is a ubiquitous stress-inducible enzyme that catalyzes the oxidative degradation of free heme. HO-1 cleaves heme to form carbon monoxide (CO), iron and biliverdin (BV), the latter being subsequently converted into bilirubin (5). HO-1 expression is up-regulated in response to various inflammatory stimuli, and this is associated with reduced inflammation. The anti-inflammatory effect of HO-1 is due to these by-products of HO-1 activity. CO is the most responsible for anti-inflammatory action and suppresses the production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and macrophage inflammatory protein-1, iNOS and cyclo-oxygenase-2 (COX-2) (6,7). BV has the anti-inflammatory effects on organ transplantation including decreased leukocyte infiltration, less T cell proliferation, and extended survival of allogeneic heart transplants (8). It reduced production of the pro-inflammatory cytokines and enhanced the anti-inflammatory cytokines in rat model (9). HO-1 gene expression is regulated by various stimuli and transcription factors. Above all, nuclear factor E2-related factor 2 (Nrf2) mainly regulates HO-1 gene expression via various intracellular signaling molecules including phosphatidylinositol 3-kinase (PI3K)/Akt pathway and mitogen-activated protein kinase (MAPK) pathway (10). In basal condition, cytoplasmic Nrf2 is associated with Kelch-like ECH associated protein 1 (Keap1) and degraded via ubiquitination. Numerous prooxidant stimuli cause dissociation of Nrf2 from Keap1, which permits subsequent nuclear translocation of Nrf2. In nucleus, Nrf2 binds to antioxidant response element (ARE) or electrophile responsive element (EpRE) of various antioxidant enzymes including HO-1, NAD(P)H:quinone oxidoreductase-1 (NQO1), and superoxide dismutase (SOD) (11).
In this study, we investigated the effects of KP on NF-κB activation and MAPKs activities which have been known to regulate NF-κB in mouse peritoneal macrophages. We also examined whether KP upregulate HO-1 expression which is responsible for anti-inflammatory effect.
MATERIALS AND METHODS
Preparation of extract
The stem bark of KP was purchased from a local herb store, Kwang Myoung Herb Medicine (Pusan, Korea) in April 2005. The roots were identified and authenticated by Professor W. S. Ko, College of Oriental Medicine, Dongeui University (Pusan, Korea). A voucher specimen (number KP-05-04) has been deposited at the Department of Molecular Biology, Pusan National University, Busan, Korea. The dry roots (300 g) were extracted with distilled water at 100℃ for 4 h. The extract was filtered through 0.45µm filter and freeze-dried (yield, 27 g) and kept at 4℃. The dried extract was dissolved in phosphate buffered saline (PBS) and filtered through 0.22 µm filter before use.
Macrophage culture
C57BL/6 mice purchased from Dae Han Laboratory Animal Center (Korea) were used between 8 to 12 weeks of age (25~30 g). Thioglycollate (TG) broth (Brewer, DIFCO, Detroit, MI)-elicited macrophages were harvested 3 days after intraperitoneal injection of 2.5 ml TG into mice and isolated as reported previously (12). Murine macrophage RAW 264.7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with glutamine (1 mM) and 10% FBS.
Measurement of nitrite concentration
NO synthesis in cell cultures was measured by a microplate assay method. To measure nitrite, 100µl aliquots were removed from conditioned medium and incubated with an equal volume of the Griess reagent (1% sulfanilamide/0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride/2.5% H3PO4) at room temperature for 10 min. Nitrite concentration was determined by measuring the absorbance at 540 nm with a Vmax 96-well microplate spectrophotometer (Molecular Devices, Menlo Park, CA). The sodium nitrite was used as a standard.
Cell viability assay
The cytotoxicity of KP was assessed using the microculture tetrazolium (MTT)-based colorimetric assay. The remaining cells after Griess reaction were used for MTT assay. 5µl of MTT solution (5 mg/ml) was added to each well (final concentration is 62.5µg/ml). After incubation for 3 h at 37℃ and 5% CO2, the supernatant was removed and the formed formazan crystals in viable cells were solubilized with 150µl of DMSO. The absorbance of each well was then read at 570 nm using microplate reader.
Preparation of nuclear extract and eletrophoretic mobility shift assay
Nuclear extracts were prepared as described previously (12). Eletrophoretic mobility shift assay (EMSA) of nuclear extracts was performed according to the manufacturer's instructions (Promega, Madison, WI) with some modifications. In brief, the probe consisted of a double-stranded oligonucleotide containing the consensus binding sequence for NF-κB (5'-AGTTGAGGGGACTTTCCCAGGC-3', Promega, Madison, WI) was end-labeled with [γ-32P]-ATP (3,000 Ci/mmol at 10µCi/ml) using T4 polynucleotide kinase. Nuclear extracts were incubated with gel shift binding buffer (10 mM HEPES, pH 7.9, 50 mM KCl, 0.2 mM EDTA, 2.5 mM DTT, 10% glycerol, 0.05% NP-40, 0.25 mg/ml poly dI/poly dC, protease inhibitor cocktail) for 10 min at room temperature and then the mixture was incubated with 32P-labeled probe for 20 min at room temperature. The incubation mixture was loaded onto non-denaturating gel and run in 0.25× Tris/borate/EDTA buffer. Gels were dried and exposed to X-ray film at -70℃.
Western blot analysis
The cells were washed with phosphate buffered saline (PBS) three times and scraped off and lysed with lysis buffer (1% Triton X-100, 1% deoxycholate). Protein concentration of lysates was determined and equal amounts of protein were separated electrophoretically using 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), and then the gel was transferred to 0.45µm nitrocellulose paper. The blot was incubated with anti-iNOS, IκB-α, HO-1, Nrf2, β-tubulin antibody (Santa Cruz Biotechnology), anti-phospho-IκB-α, JNK, phospho-JNK, ERK1/2, phospho-ERK1/2 antibody (Cell Signaling Technology), and secondary antibody and then detected by the enhanced chemiluminescence detection system according to the recommended procedure (Amersham Co.).
RESULTS
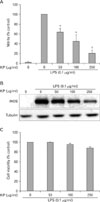
KP inhibits NO synthesis and iNOS expression in macrophages
To investigate the anti-inflammatory effect of KP, we examined the effect of KP on NO synthesis. TG-elicited mouse peritoneal macrophages were incubated with KP for 1 h and stimulated with 0.1µg/ml LPS for 24 h. The amount of NO released into culture medium was measured by the method of Griess. Whereas LPS-treated cells produced a large amount of NO, KP suppressed NO release into culture supernatant in a dose-dependent manner (Fig. 1A). To determine whether the decreased nitric oxide synthesis is correlated with iNOS expression, we analyzed the amount of iNOS by Western blot analysis. TG-elicited mouse peritoneal macrophages were treated with KP as mentioned above. The level of iNOS was dramatically reduced by KP in a dose-dependent manner (Fig. 1B). On the other hand, cell viability was not affected by KP as measured by MTT assay (Fig. 1C). These results suggest that KP inhibits NO release by affecting the iNOS expression level without affecting cell viability.
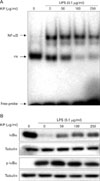
KP suppresses NF-κB activity and phosphorylation/degradation of IκB-α in macrophages
Since iNOS promoter contains 2 binding sites for transcription factor NF-κB and NF-κB plays a critical role in LPS-induced iNOS expression, we investigated whether KP suppresses iNOS expression through the regulation of NF-κB activity. TG-elicited mouse peritoneal macrophages were incubated with KP for 1 hr and stimulated with 0.1µg/ml LPS for 30 minutes. A low level of NF-κB/DNA complex was observed in unstimulated macrophages, while a large quantity of NF-κB/DNA complex was induced by LPS treatment. This increased NF-κB binding affinity was inhibited by KP in a dose-dependent manner (Fig. 2A). These results suggest that KP suppresses iNOS expression though inhibition of NF-κB activity.
To analyze whether KP affect NF-κB signaling pathway, we investigated the effects of KP on upstream signaling pathway of NF-κB, that is, IκB-α degradation. TG-elicited mouse peritoneal macrophages were pretreated with KP for 1 h and stimulated with LPS (0.1µg/ml) for 15 min, and then the levels of IκB-α or phospho-IκB-α protein were examined by Western blotting. As shown in Fig. 2B, IκB-α was degraded in response to LPS, but KP suppressed the degradation of IκB-α in a dose-dependent manner. Incubation of macrophages with LPS for 15 min also caused significant phosphorylation of IκB-α, but KP markedly inhibited the phosphorylation of IκB-α. These results indicate that KP blocks NF-κB translocation into nucleus through suppression of IκB-α phosphorylation and degradation.
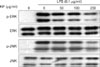
KP inhibits LPS-induced MAPK activities
To elucidate the molecular target of KP in further upstream signaling pathway, we examined the effect of KP on MAPKs activities which regulate the function of NF-κB (13). Since extracellular signal-regulated kinase1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) have been known to be phosphorylated and activated in response to LPS, the activities were monitored by its phosphorylation. TG-elicited mouse peritoneal macrophages were treated with indicated concentrations of KP for 1 h and then stimulated with 0.1µg/ml LPS for 15 min. KP suppressed LPS-induced activation of ERK1/2 and JNK in a dose-dependent manner (Fig. 3), while the amount of kinases was unaffected by either LPS or KP treatment.
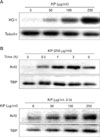
HO-1 expression is induced by KP in macrophages
It is known that by-products of HO-1 have anti-inflammatory effect. To determine whether KP exhibit anti-inflammatory effect through the induction of HO-1, HO-1 expression by KP was examined. TG-elicited mouse peritoneal macrophages were treated with KP for 24 h. KP induced HO-1 expression in a dose-dependent manner (Fig. 4A).
To determine whether KP-induced HO-1 expression is caused by Nrf2 transcription factor, we examined the effect of KP on Nrf2 nuclear translocation. TG-elicited mouse peritoneal macrophages were treated with KP and cytosolic and nuclear extracts were separately harvested. The amount of nuclear Nrf2 was increased by KP in dose-dependent and time-dependent manners, whereas the amount of cytosolic Nrf2 did not showed prominent changes (Fig. 4B).
DISCUSSION
The application of medicinal herbs dates back to the beginning of civilization, and interestingly, medicinal herbs are still routinely used by most of world's population. Over time, many information has been accumulated by using these herbs in practice, and it is experientially proved that these herbs have the effect of pain alleviation, life extension and disease prevention. Of these, the stem bark of KP has been used for different remedies and expresses specific therapeutic effects in China and Korea. The stem bark of KP is mainly used for treating neuralgia, rheumatic arthritis, lumbago, furuncle, carbuncle, wound, diarrhea and scabies (2). It has been reported that hederagenin monodesmosides and bisdesmosides isolated from the stem bark of KP have antimutagenic and cytotoxic activity (14). Kalopanaxsaponin A and pictoside A from the stem bark of KP reduce vascular permeability (2). The other constituent of KP, α-hederin methyl ester, showed anticarrageenan activity in rats and anti-arthritic activity in rats and mice (15). Kalopanaxsaponin A was reported to have anti-rheumatoidal (16-19), anti-tumor (20), anti-diabetic (21), antifungal (22) activities. Kalopanaxsaponins A inhibits the production of NO, prostaglandin E2 (PGE2) and TNF-α in macrophage cell line RAW 264.7 (1). In this study, KP significantly inhibited LPS-induced NO production and iNOS expression in mouse peritoneal macrophages (Fig. 1), which is consistent with the results of Kim et al. (1). Because KP did not show cytotoxic effects, inhibition of iNOS expression might be specific. Major transcriptional regulators of iNOS gene are the NF-κB family of transcription factors, which are also key regulators of a variety of genes involved in immune and inflammatory responses. Since dysregulation of NF-κB function is associated with inflammation, a development of drug that control NF-κB is one of promising candidates for the therapeutic strategy in the treatment of inflammatory disease. Our study showed that KP significantly inhibited NF-κB DNA binding activity in macrophages. Although it was reported that the combined extracts containing KP and other herb medicine suppress NF-κB DNA binding activity in RAW 264.7 cells (23), the effect of KP on NF-κB activity has remained to be clarified. Moreover, our study showed that KP suppressed not only the LPS-stimulated degradation but also phosphorylation of IκB-α. The phosphorylation of N-terminal regulatory serines on IκB-α and IκB-α is known to be catalyzed by IKK. Thus, KP could regulate the IKK activity. Since MAPKs have been shown to be involved in the LPS-mediated induction of many inflammatory genes including iNOS gene and are important for the activation of NF-κB (13), we investigated the effect of KP on ERK1/2 and JNK activation in LPS-stimulated mouse peritoneal macrophages. KP greatly inhibited the LPS-induced ERK1/2 and JNK after LPS application. These results suggest that KP inhibits LPS-induced NF-κB activation by down-regulating ERK1/2 and JNK.
HO breaks down free heme made from intracellular heme-containing proteins. Two types of isozyme have been identified; HO-1 and 2. Whereas HO-2 is a constitutively expressed form, HO-1 is an inducible form that expressed by either inflammatory or oxidative stress (5). Recently, many reports showed that HO-1 expression is associated with reduced inflammation. HO-1 plays a critical protective role during the development of intestinal inflammation (24,25). HO-1 attenuates metabolic syndrome in obese mice (26), LPS-induced lung injury (27), cardiovascular desease (28) and liver desease (29). HO-1-deficient mice develop increased end-organ damage and have increased mortality after LPS administration (30). Human HO-1 deficiency presents with severe hemolysis, asplenia, inflammation and nephritis (31). In addition, LPS-induced iNOS expression is reduced by HO-1 expression (24). In this study, HO-1 expression was increased by KP, and nuclear translocation of Nrf2, a major transcription factor of HO-1 expression, also induced by KP. These results suggest that KP exhibit anti-inflammatory effect by suppressing NO production via HO-1 induction.
In conclusion, we demonstrated that KP inhibited NO release and iNOS expression in LPS-stimulated macrophages, and that these effects are mediated by inhibition of the activity of IκB/NF-κB and induction of HO-1. Our results provide a molecular basis for understanding the inhibitory effects of KP on endotoxin-mediated inflammation. As the effect of KP is mediated via inhibition of NF-κB and HO-1 induction, KP could be developed into potent anti-inflammatory drugs and be used in pathological processes in which NF-κB and HO-1 are involved.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download