Abstract
Background
Lichen-derived glucans have been known to stimulate the functions of immune cells. However, immunostimulatory activity of glucan obtained from edible lichen, Umbilicaria esculenta, has not been reported. Thus we evaluated the phenotype and functional maturation of dendritic cells (DCs) following treatment of extracted glucan (PUE).
Methods
The phenotypic and functional maturation of PUE-treated DCs was assessed by flow cytometric analysis and cytokine production, respectively. PUE-treated DCs was also used for mixed leukocyte reaction to evaluate T cell-priming capacity. Finally we detected the activation of MAPK and NF-κB by immunoblot.
Results
Phenotypic maturation of DCs was shown by the elevated expressions of CD40, CD80, CD86, and MHC class I/II molecules. Functional activation of DCs was proved by increased cytokine production of IL-12, IL-1β, TNF-α, and IFN-α/β, decreased endocytosis, and enhanced proliferation of allogenic T cells. Polymyxin B, specific inhibitor of lipopolysaccharide (LPS), did not affect PUE activity, which suggested that PUE was free of LPS contamination. As a mechanism of action, PUE increased phosphorylation of ERK, JNK, and p38 MAPKs, and enhanced nuclear translocation of NF-κB p50/p65 in DCs.
In recent decades, glucan polysaccharides isolated from botanical sources, including plants, mushrooms, algae, and lichens, have attracted a great deal of attention in the biomedical area because of their broad spectrum of therapeutic properties and relatively low toxicities (1-3). While the mechanism of action is still unclear, it appears that one of the primary mechanisms involves nonspecific activation of the immune system (2). Indeed, the basic mechanism of the immunostimulatory, anti-tumor, anti-infection, and other therapeutic effects of glucans is thought to occur via the modulation of innate immunity including macrophage and dendritic cell (DC) functions (1).
The majority of glucans were isolated from mushrooms, such as lentinan from lentinus eodes (4), schzophyllan from Schizophyllum commune (5), krestin from Coriolus versicolor (6), grifolan from Grifola frondosa (7), and scleroglucan from Sclerotinia sclerotiorum (8). Similarly, glucans were isolated from lichens, including lichenan and isolichenan from Cetraria islandica (9-11) and thamnolan from Thamnolia subuliformis (11-13). Lichen-derived glucans stimulated immune functions of DCs, macrophages, and spleen cells (10,12). In this study, we investigated immunomodulating activity of glucan isolated from an edible lichen Umbilicaria esculenta, which had been used for a traditional food or a medicine in the Far East such as Korea, Japan and China (14). Extracts or prescriptions of Umbilicaria esculenta showed inhibitory activities on thrombosis (14), melanogenesis (15), and glycosidase (16). However, immunomodulatory activity of glucan from U. esculenta has not been reported yet. We investigated here the immunostimulatory activity of glucan isolated from U. esculenta, especially focusing on DC functions.
Female C57BL/6 and BALB/c mice (6~8 weeks old) were obtained from Korea Research Institute of Bioscience and Biotechnology (Chungbuk, Korea). Mice were housed in specific pathogen-free conditions at 21~24℃ and 40~60% relative humidity under a 12-h light/dark cycle. All animals were acclimatized for at least 1 week prior to the experiments. The experimental procedures used in this study were approved by the Animal Experimentation Ethics Committee in Chungbuk National University. Antibodies against mouse CD11c, CD40, CD80, CD86, and MHC class I/II were purchased from BD Pharmingen (San Diego, CA, USA), and those against ERK, p38, JNK, p65, and p50 were purchased from Cell Signaling Technology (Beverly, MA, USA).
PUE was extracted from an edible lichen U. esculenta. Briefly, the dried U. esculenta was pulverized into powder. The sample was then defatted three times with dichloromethane. After centrifugation, the residue was successively extracted twice with hot water (100℃) each time for 2 h. The extract was combined and concentrated under reduced pressure to small volumes. The crude polysaccharide was precipitated by adding two volumes of ethanol. The precipitate was collected by centrifugation and washed twice with ethanol. The precipitate was then suspended in water and lyophilized to yield crude glucan, named as PUE. PUE included 70.8% of immunostimulating glucan (Megazyme, Wicklow, Ireland). No endotoxin was detected at concentrations up to 500 µg/ml PUE, which was determined by LAL test (Catalogue number 291-53101, Wako Pure Chemicals, Osaka, Japan).
RAW264.7 macrophages were plated at 5×105 cells/ml and then stimulated with PUE or LPS for 24 h. Isolated supernatants were mixed with an equal volume of Griess reagent and then incubated at room temperature for 10 min. Nitrite production was determined by measuring absorbance at 540 nm versus a NaNO2-derived standard curve (17).
Spleen cells were obtained from a specific pathogen-free C57BL/6 mouse (female, 6~7 weeks old) and freed of red blood cells by treating with lysis buffer. Spleen cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA, USA), 2 mM glutamine and 50 µM 2-mercaptoethanol (Sigma, St. Louis, MO, USA). Cells were seeded in a 96-well, flat-bottomed plate at a density of 2×105 cells/well and stimulated with PUE or lipopolysaccharide (LPS) (17). Cells were pulsed with 3H-thymidine (113 Ci/nmol, NEN, Boston, MA, USA) at a concentration of 1 µCi/well for the last 18 h and harvested on day 3 using an automated cell harvester (Inotech, Dottikon, Switzerland). The amount of 3H-thymidine incorporated into the cells was measured using a Wallac Microbeta scintillation counter (Wallac, Turku, Finland).
DCs were generated from bone marrow (BM) cells obtained from 6~7-week-old female mice (18). Briefly, BM cells were flushed out from femurs and tibias. After red blood cells were lysed, whole BM cells (2×105 cells/ml) were cultured in 100-mm2 culture dishes in 10 ml/dish of complete medium containing 2 ng/ml GM-CSF (R&D Systems, Minneapolis, MN, USA). On day 3, another 10 ml of fresh complete medium containing 2 ng/ml GM-CSF was added, and half of the medium was changed on day 6. On day 8, non-adherent and loosely adherent DCs were harvested by vigorous pipetting and used as immature DCs (iDCs). iDCs recovered from these cultures were generally >85% CD11c+, but not CD3+ and B220+.
Phenotypic maturation of DCs was analyzed by flow cytometry (19). Cell were stained using a combination of FITC-conjugated anti-CD40, anti-CD80, anti-CD86, or anti-MHC plus PE-conjugated CD11c antibodies. Cells were analyzed using a FACSCanto flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed using WinMdi software (Scripps, La Jolla, CA, USA). Forward and side scatter parameters were used to gate live cells. Cell viability was examined by the propidium iodide (PI) nuclear staining method. Cells were stained with 1 µg/ml of PI and analyzed with a FACSCanto flow cytometer. Cells stained with PI were considered dead cells.
To analyze the endocytosis of DCs, 4×105 DCs were incubated at 37℃ for 1 h with 0.7 mg/ml FITC-dextran (42,000 Da, Sigma-Aldrich, St. Louis, MO, USA). After incubation, cells were washed twice with cold washing buffer (PBS containing 0.5% BSA) and stained using PE-conjugated anti-CD11c antibody. Double-stained DCs were analyzed by flow cytometry. Parallel experiments were performed at 4℃ to determine the nonspecific binding of FITC-dextran to DCs (20).
Total RNA was isolated using TRIZOL™ Reagent (Molecular Research Center, Cincinnati, OH, USA). For RT-PCR, single-strand cDNA was synthesized from 2 µg total RNA. The primer sequences used were as follows (20): IL-12, sense, 5'-AGA GGT GGA CTG GAC TCC CGA-3', antisense, 5'-TTT GGT GCT TCA CAC TTC AG-3'; TNF-α, sense, 5'-AGG TTC TGT CCC TTT CAC TCA CTG-3', antisense, 5'-AGA GAA CCT GGG AGT CAA GGT A-3'; IL-1β, sense, 5'-ATG GCA ATG TTC CTG AAC TCA ACT-3', antisense, 5'-CAG GAC AGG TAT AGA TTC TTT CCT TT-3'; IFN-α, sense, 5'-TCT GAT GCA GCA GGT GGG-3', antisense, 5'-AGG GCT CTC CAG AYT TCT GCT CTG-3'; IFN-β, sense, 5'-CCA CAG CCC TCT CCA TCA ACT ATA AGC-3', antisense, 5'-AGC TCT TCA ACT GGA GAG CAG TTG AGG-3'; β-actin, sense, 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3', and antisense 5'-TAA AAC GCA GCT CAG TAACAG TCC G-3'. PCR products were fractionated on 1% agarose gels and stained with 5 µg/ml ethidium bromide. After analyzing band areas using an ImageJ analysis system (ImageJ, NIH, MD, USA), target mRNA expression levels were calculated as relative ratios versus β-actin. Cytokine levels of IL-2 and IFN-γ in culture supernatants were measured using commercial immunoassay kits (R&D Systems, Minneapolis, MN, USA).
Cell lysates were prepared as previously described (18). Detergent-insoluble materials were removed, and equal amounts of protein were fractionated by 10% SDS-PAGE and transferred to pure nitrocellulose membranes. Membranes were blocked with 5% BSA in Tween 20 plus TBS (TTBS) for 1 h and then incubated with an appropriate dilution of primary antibody in 5% BSA (in TTBS) for 2 h. Blots were incubated with biotinylated antibody for 1 h and further incubated with HRP-conjugated streptavidin for 1 h. Signals were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Responder T cells were purified from the spleen of BALB/c mice by negative depletion using biotinylated antibodies against B220, GR-1, and CD11c (BD Pharmingen, San Diego, CA, USA) and Dynabeads M-280 streptavidin (Invitrogen, Carlsbad, CA, USA), as previously described (18). Purity was typically more than 90%. DCs generated from BM cells of C57BL/6 mice were treated with 40 µg/ml mitomycin C (MMC) for 1 h. MMC-treated DCs from 300 to 10,000 cells were added to 1×105 T cells in U-bottom 96-well plates (activator: responder cell ratio=0.3-10). Allogenic T cells were pulsed with [3H]-thymidine (113 Ci/nmol, NEN, Boston, MA, USA) at a concentration of 1 µCi/well for the last 18 h and then harvested on days 3 and 5 using an automated cell harvester (Innotech, Dottikon, Switzerland). The amount of [3H]-thymidine incorporated into the cells was measured using a Wallac Microbeta scintillation counter (Wallac, Turku, Finland).
First, we investigated the immunostimulating activities of PUE on several immune cells including DCs, macrophages, and spleen cells (mainly including B and T cells) to determine cell-type selectivity. PUE increased NO production by RAW264.7 macrophages (Fig. 1A), spleen cell proliferation (Fig. 1B), and MHC class I expression in BM-derived DCs (Fig. 1C). LPS was used as a positive activator of B cells, macrophages, and dendritic cells. This result suggests that PUE increases immune functions of macrophages, splenic lymphocytes, and DCs. Subsequently, we investigated the stimulating effect of PUE on maturation and function of DCs in greater detail, since they were considered as main regulatory cells connecting innate and adoptive immunity.
We examined the phenotypic maturation of DCs by determining the expression level of MHC class II, CD40, and CD80/86, which were involved in T cell activation. PUE increased the expression of MHC class II (Fig. 2A), CD40 (Fig. 2B), CD80 (Fig. 2C), and CD86 in dose-dependent manner (Fig. 2D). PUE- or LPS-treated DCs showed a mature morphology with long dendrites, but untreated DCs had short dendrites. Mature DCs were increased in size and granularity, as indicated by the forward- and side-scatter values on flow cytometry. PUE or LPS did not affect cell viability during the incubation (data not shown). These results demonstrate that PUE induces the phenotypic maturation of DCs.
The field of glucan research has been confounded by the presence of the endotoxin LPS in glucan preparations. To rule out the possibility of LPS contamination in PUE, we treated PUE with polymyxin B (PMB), a specific inhibitor of LPS. PMB strongly inhibited MHC class II expression by LPS-treated DCs, but not by PUE-treated DCs (Fig. 2E). This result suggests that the PUE was free of LPS contamination and that the DC maturation was glucan specific.
Cytokine expression is a parameter of functional maturation of DCs. PUE strongly increased IL-12 gene expression by DCs, which is a major factor in the induction of Th1 immune response (Fig. 3A). In addition, PUE increased gene expression of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IFN-α/β by DCs. Immature DCs efficiently capture antigens and show a high level of endocytosis, but mature DCs have reduced antigen capture capacities. PUE-treated DCs showed reduction of antigen-uptake in dose-dependent manner (Fig. 3B). A similar result was obtained with LPS-treated DCs. Parallel experiments were performed at low temperature to rule out nonspecific binding of dextran-FITC to DCs. Antigen uptake by DCs was inhibited at low temperature, which suggested that DC endocytosis was a specific, active process. These results demonstrate that PUE induces the functional maturation of DCs.
The phenotypic and functional maturations of DCs by PUE suggest that PUE-treated DCs might play a role as stimulator cells during allogenic T cell responses. To test this hypothesis, we induced mixed leukocyte response using C57BL/6 mouse-derived DCs (H-2b) and BALB/c mouse-derived T cells (H-2d). After three (Fig. 4A) or five days (Fig. 4B), PUE-treated DCs strongly enhanced allogenic T cell proliferation, but immature DCs slightly affected this response. T cells or MMC-treated DCs alone could not proliferate. These results suggest that PUE-treated DCs have high capacity to stimulate allogenic T cells to proliferate and produce cytokines.
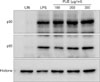
Signaling pathways involving NF-κB and MAPK play a major role in DC maturation. Thus, we investigated the activation of MAPKs and NF-κB in PUE-treated DCs. As shown in Fig. 5, basal levels of phosphorylated ERK, JNK, and p38 MAPKs in immature DCs were low, whereas phosphorylation of MAPKs were profoundly increased over basal levels in DCs upon exposure to either LPS or PUE, indicating that MAPK pathways might be involved in PUE-induced DC maturation. The majority of NF-κB subunits are sequestered in the cytoplasm by IκBα/β and translocated into the nucleus after IκBα/β degradation. As shown in Fig. 6, PUE induced the nuclear translocation of NF-κB p50 and p65, as demonstrated by the increased levels of nuclear p50/p65 proteins. These results suggested that PUE induced DC maturation through MAPK and NF-κB signalings.
U. esculenta is an edible and medicinal lichen containing large amounts of β-1,3-glucan (PUE) and we show here that PUE has immunostimulating activities. In general, antitumor immunity in physiological conditions is coordinated by both innate and adaptive immunity, and is mainly mediated by diverse immune effector cells, such as DCs, macrophages, T cells, B cells, and others. In this study, we examined the direct effect of PUE on immune effector cells and we found that PUE activated DCs and macrophages, and spleen cells (mainly consisting of B and T cells). In the subsequent studies, we tried to investigate the effect of PUE on DCs in greater detail, since they are main regulating players, which can induce, coordinate, and regulate antitumor immunity (21). DCs are professional antigen-presenting cells that are crucial for the initiation of tumor-specific T cell responses (21). DCs are scattered through the body as immature cells with potent antigen-uptake functions. On receiving cytokines or other inflammatory mediators, they mature and migrate to the T cell zones of draining lymph nodes, and sensitize T cells through major histocompatibility (MHC) and co-stimulatory molecules (22). This fact suggests that a maturation step is essential for DCs to initiate T cell activation. Especially, DC maturation is a critical step in inducing anti-tumor immunity. Solid tumors contain small number of DCs, and most of them are immature cells (23). These immature DCs cannot induce antitumor immune responses (24). In addition, it has been reported that tumors prevent DC maturation and functions by releasing immunosuppressive factors, such as VEGF, PGE2, and IL-10 (25-30). During the past several decades, many studies have attempted to find immunomodulators that induce DC maturation. In particular, plant-derived polysaccharides have been suggested as promising candidates for DC maturation. For example, glucan polysaccharides isolated from Phellinus linteus, Agaricus blazei, Coriolus versicolor, Cordyceps militaris, and Angelica gigas have been found to induce phenotypic and functional maturation of murine immature DCs (19, 31-34). Here, we demonstrate that PUE from U. esculenta might also be a good inducer of DC maturation.
Several phenotypic and functional changes were observed in PUE-treated DCs. PUE increased expressions of MHC class I/II and of co-stimulatory molecules (CD40 and CD80/86), resulting in enhanced antigen presentation to T cells (35). PUE also increased cytokine production by DCs. Among DC-derived cytokines, IL-12 is the main cytokine that can activate Th1 cells. Activated Th1 cells can produce IFN-γ and IL-2, which can activate antitumor effector cells, such as cytotoxic T cells and NK cells (36). In addition, other inflammatory cytokines (TNF-α and IL-1β) produced by DCs can up-regulate adhesion molecules by the endothelium and these molecules contribute to the recruitment of monocytes and other cell types to the tumor microenvironment (37). These results suggest that PUE activated DCs and resulted in the overall activation of antitumor effector cells in tumor environments. PUE-induced DC maturation is likely to involve at least two important signaling pathways: MAPKs and NF-κB/Rel. This coordinated process in DCs leads to IL-12 release and to a high T cell-stimulatory capacity, both of which result in the induction of a protective immune response. Our results show that PUE induces DC maturation through MAPK and NF-κB activation.
One question raised in this study might be whether PUE was free of LPS contamination. The field of glucan research has been confounded by the presence of the endotoxin LPS in glucan preparations. In this study, to rule out the possibility of LPS contamination in PUE, we validated PUE purity in two different ways. First, we treated PUE with polymyxin B (PMB), a specific inhibitor of LPS. PMB strongly inhibited MHC class II expression by LPS-treated DCs, but not by PUE-treated DCs. Second, we examined endotoxin levels in PUE by using Limulus Amebocyte Lysate (LAL) test and proved that no endotoxin was detected at concentrations up to 500 µg/ml PUE. This result suggested that the PUE was free of LPS contamination and that DC maturation was β-glucan specific.
The body's natural defenses are usually attenuated in cancer patients. In particular, DCs that are infiltrated into tumor tissues show reduced expression of co-stimulatory molecules and defective cytokine production, which implies that tumor-derived factors impede DC maturation (22,23). Defective DCs in tumor microenvironments is an important immunological problem that limits the success of cancer immunotherapy. In the present study, we find that PUE can induce the phenotypic and functional maturation of DCs through MAPK and NF-κB signaling, which suggests that PUE might increase the efficiency of DC-based cancer immunotherapy.
Figures and Tables
Figure 1
Cell-type selectivity of PUE. RAW 264.7 macrophages were treated with PUE for 24 h and NO production was determined (A). Spleen cells were treated with PUE for 72 h and lymphoproliferation was determined (B). Immature DCs were treated with PUE for 24 h and MHC class I expression level was determined (C). LPS (1 µg/ml) was used as a general activator of DCs, RAW 264.7 cells, and B cells. Significance was determined using the ANOVA test versus chemically-untreated control groups (UN) (*p<0.01).

Figure 2
Phenotypic maturation of DCs by PUE. Immature DCs were treated with LPS (1 µg/ml) or PUE. After incubation for 24 h, DCs were stained with PE-conjugated CD11c Ab plus FITC-conjugated Abs against MHC class II (A), CD40 (B), CD80 (C), and CD86 (D). CD11c-PE was used to gate live DCs. Results are presented as mean±STD. In another experiment, PUE or LPS was pre-treated with 1,000 units/ml PMB for 2 h or left untreated, and then incubated with immature DCs. After incubation for 24 h, cells were stained with PE-conjugated CD11c Ab plus FITC-conjugated MHC class II Ab (E). Mean fluorescence intensities (MFI) of three separate experiments are shown. Significances were determined using the ANOVA test versus chemically-untreated control groups (UN) (*p<0.01) and versus PMB-untreated group (†p<0.01).

Figure 3
Effect of PUE on cytokine gene expression of DCs. (A) Immature DCs were activated with LPS or PUE for 4 h. Then, total RNA was isolated and RT-PCR was performed to examine gene expression levels of IL-12, IL-1β, TNF-α, and IFN-α/β. (B) Immature DCs were activated with LPS or PUE for 24 h and then treated with 0.7 mg/ml of dextran-FITC for 1 h at 37℃. After washing, DCs were stained with PE-conjugated anti-CD11c Ab and double-stained DCs were analyzed by flow cytometry. Parallel experiments were performed at 4℃ to confirm nonspecific binding of FITC-dextran. Mean fluorescence intensities (MFI) of three separate experiments are shown. Significances were determined using the ANOVA test versus chemically-untreated control groups (UN) (*p<0.01).

Figure 4
Allo-stimulation of T cells using PUE-treated DCs. Immature DCs were activated with LPS or PUE for 24 h, and treated with 40 µg/ml mitomycin C (MMC) for 1 h to prevent proliferation. MMC-treated DCs (300~10,000) were added to 1×105 T cells per well. After incubation for 3 (A) and 5 days (B), mixed cell populations were labeled with [3H]-thymidine and harvested using an automated cell harvester.

Figure 5
Effect of PUE on MAPK signalings. iDCs were generated from mouse BM cells by treating them with 2 ng/ml of GM-CSF for 8 days, and then further activated with LPS (1 µg/ml) or PUE for 15 min. Total cell extracts were isolated and blotted with anti-phospho-ERK, -JNK, and -p38 antibodies. UN, chemically-untreated control groups.
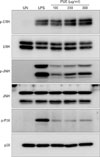
Figure 6
Effect of PUE on NF-κB signalings. iDCs were generated from mouse BM cells by treating them with 2 ng/ml of GM-CSF for 8 days, and then further activated with LPS (1 µg/ml) or PUE for 6 h and nuclear extracts were blotted with anti-p50 and anti-p65 antibodies. UN, chemically-untreated control groups.
ACKNOWLEDGEMENTS
This research was supported by the Medical Research Center program (2010-0029480) through the National Research Foundation of Korea and by the Research & Development for Regional Industry program (70004559) through the Korea Institute for Advancement of Technology.
References
1. Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006. 6:317–333.

2. Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000. 13:523–533.

3. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002. 60:258–274.

4. Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. Kanagawa Lentinan Research Group. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Hepatogastroenterology. 1999. 46:2662–2668.
5. Okamura K, Suzuki M, Chihara T, Fujiwara A, Fukuda T, Goto S, Ichinohe K, Jimi S, Kasamatsu T, Kawai N, et al. Clinical evaluation of schizophyllan combined with irradiation in patients with cervical cancer. A randomized controlled study. Cancer. 1986. 58:865–872.

6. Pang ZJ, Zhou M, Chen Y, Wan J. A protein-bound polysaccharide synergistic with lipopolysaccharide induces nitric oxide release and antioxidant enzyme activities in mouse peritoneal macrophages. Am J Chin Med. 1998. 26:133–141.

7. Okazaki M, Adachi Y, Ohno N, Yadomae T. Structure-activity relationship of (1-->3)-beta-D-glucans in the induction of cytokine production from macrophages, in vitro. Biol Pharm Bull. 1995. 18:1320–1327.

8. Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999. 221:281–293.

9. Ingolfsdottir K, Jurcic K, Fischer B, Wagner H. Immunologically active polysaccharide from Cetraria islandica. Planta Med. 1994. 60:527–531.

10. Freysdottir J, Omarsdottir S, Ingólfsdóttir K, Vikingsson A, Olafsdottir ES. In vitro and in vivo immunomodulating effects of traditionally prepared extract and purified compounds from Cetraria islandica. Int Immunopharmacol. 2008. 8:423–430.

11. Olafsdottir ES, Ingolfsdottir K, Barsett H, Paulsen BS, Jurcic K, Wagner H. Immunologically active (1-->3)-(1-->4)-alpha-D-glucan from Cetraria islandica. Phytomedicine. 1999. 6:33–39.

12. Omarsdottir S, Freysdottir J, Olafsdottir ES. Immunomodulating polysaccharides from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2007. 14:179–184.

13. Olafsdottir ES, Omarsdottir S, Paulsen BS, Wagner H. Immunologically active O6-branched (1-->3)-beta-glucan from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2003. 10:318–324.

14. Kim MS, Lee KA. Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J Ethnopharmacol. 2006. 105:342–345.

15. Kim MS, Cho HB. Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol. 2007. 45:578–582.
16. Lee KA, Kim MS. Glucosidase inhibitor from Umbilicaria esculenta. Can J Microbiol. 2000. 46:1077–1081.
17. Kim HS, Kim JY, Ryu HS, Park HG, Kim YO, Kang JS, Kim HM, Hong JT, Kim Y, Han SB. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int Immunopharmacol. 2010. 10:1284–1294.

18. Kim JY, Kang JS, Kim HM, Ryu HS, Kim HS, Lee HK, Kim YJ, Hong JT, Kim Y, Han SB. Inhibition of bone marrow-derived dendritic cell maturation by glabridin. Int Immunopharmacol. 2010. 10:1185–1193.

19. Kim JY, Yoon YD, Ahn JM, Kang JS, Park SK, Lee K, Song KB, Kim HM, Han SB. Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. Int Immunopharmacol. 2007. 7:78–87.

20. Kim HS, Kim JY, Kang JS, Kim HM, Kim YO, Hong IP, Lee MK, Hong JT, Kim Y, Han SB. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food Chem Toxicol. 2010. 48:1926–1933.

21. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.

22. Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008. 272:186–196.

23. Bennaceur K, Chapman JA, Touraine JL, Portoukalian J. Immunosuppressive networks in the tumour environment and their effect in dendritic cells. Biochim Biophys Acta. 2009. 1795:16–24.

24. Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992. 356:607–609.

25. Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996. 170:111–119.
26. Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006. 25:323–331.

27. Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998. 92:4778–4791.

28. Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004. 172:464–474.

29. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007. 25:267–296.

30. Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM Jr. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001. 61:363–369.
31. Kanazawa M, Mori Y, Yoshihara K, Iwadate M, Suzuki S, Endoh Y, Ohki S, Takita K, Sekikawa K, Takenoshita S. Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol Lett. 2004. 91:229–238.

32. Kim GY, Han MG, Song YS, Shin BC, Shin YI, Lee HJ, Moon DO, Lee CM, Kwak JY, Bae YS, Lee JD, Park YM. Proteoglycan isolated from Phellinus linteus induces toll-like receptors 2- and 4-mediated maturation of murine dendritic cells via activation of ERK, p38, and NF-kappaB. Biol Pharm Bull. 2004. 27:1656–1662.

33. Kim GY, Lee MY, Lee HJ, Moon DO, Lee CM, Jin CY, Choi YH, Jeong YK, Chung KT, Lee JY, Choi IH, Park YM. Effect of water-soluble proteoglycan isolated from Agaricus blazei on the maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2005. 5:1523–1532.

34. Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006. 119:254–264.

35. Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996. 153:85–106.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download