Abstract
Background
APRIL, originally known as a cytokine involved in B cell survival, is now known to regulate the inflammatory activation of macrophages. Although the signal initiated from APRIL has been demonstrated, its role in cellular activation is still not clear due to the presence of BAFF, a closely related member of TNF superfamily, which share same receptors (TACI and BCMA) with APRIL.
Methods
Through transfection of siRNA, BAFF-deficient THP-1 cells (human macrophage-like cells) were generated and APRIL-mediated inflammatory activities were tested. The expression patterns of APRIL were also tested in vivo.
Results
BAFF-deficient THP-1 cells responded to APRIL-stimulating agents such as monoclonal antibody against APRIL and soluble form of TACI or BCMA. Furthermore, co-incubation of the siBAFF-deficient THP-1 cells with a human B cell line (Ramos) resulted in an activation of THP-1 cells which was dependent on interactions between APRIL and TACI/BCMA. Immunohistochemical analysis of human pathologic samples detected the expression of both APRIL and TACI in macrophage-rich areas. Additionally, human macrophage primary culture expressed APRIL on the cell surface.
As members of the TNF superfamily (TNFSF), APRIL (TNFSF13) and its close relative BAFF (the B-cell activation factor of the TNF family, TALL-1, THANK, BlyS, TNFSF13b, zTNF-4) are type II membrane proteins with a short cytoplasmic region, a transmembrane domain and an extracellular domain consisting of a stalk and a TNF domain (1-4). Expression of both APRIL and BAFF were detected in various cell types including myeloid cells (monocytes, macrophages, neutrophils, and dendritic cells), stromal cells within lymphoid organs, and osteoclasts (5-8). Mouse models either transgenic or deficient in APRIL or BAFF revealed the essential role of these molecules in B-cell survival, T cell co-stimulation, autoimmune diseases and cancer (4,6-8). BAFF and APRIL share two receptors, TACI (transmembrane activator and CAML [a calcium-modulating cyclophilin ligand] interactor) and BCMA (B-cell maturation antigen), while BAFF-R (BAFF receptor, BR3) is recognized only by BAFF (6,8). Recently, both BAFF and APRIL has been demonstrated to induce so-called 'reverse signaling' when membrane-bound form of these molecules were stimulated with proper agents such as soluble form of their counterparts or agonistic monoclonal antibodies (mAbs) against them (9,10). Upon activation through BAFF or APRIL, macrophages undergo inflammatory changes which culminate at the induction of inflammatory mediators such as matrix degrading enzymes and pro-inflammatory cytokines.
Since APRIL and BAFF shares two of the three receptors, detailed analysis of the activity of APRIL is difficult to achieve. In an effort to study the role of APRIL in the inflammatory activation of macrophages in detail, BAFF expression was suppressed by specific siRNA. The resulting BAFF-deficient cells were stimulated with agents that can interact with APRIL or by direct incubation with cells that express the counterparts of APRIL. Additionally, expression patterns of APRIL were tested in pathologic samples obtained from patients and primary macrophages in order to confirm its expression in vivo.
The human monocytic leukemia cell line, THP-1, were obtained from the American Type Culture Collection (Rockville, MD, USA). Ramos cell line was a generously provided by Dr. Young-Hwa Jung (Busan University). The mAbs for APRIL (clone ab16088) was purchased from Abcam (Cambridge, MA, USA); mAb against CD68 (clone KP1) was purchased from DAKO Glostrup, Denmark); mAbs for BAFF (clone 121808) or TACI (clone 165609) and the fusion protein containing an extracellular domain of hBCMA and the Fc portion of human IgG was obtained from R&D Systems (Minneapolis, MN, USA); mAb for BCMA (clone Vicky-1) and TACI:Fc came from Alexis (San Diego, CA, USA); PD08059 and U0126 originated from Cell Signaling (Danvers, MA, USA); human IgG, SB203580, and LY294002 were obtained from Calbiochem International Inc. (La Jolla, CA, USA); and PMA, TPCK, ethyl pyruvate and sulfasalazine were purchased from Sigma (St. Luis, MO, USA).
BAFF-specific siRNAs which contain a pool of 4 siRNAs for BAFF and control siRNA which contains random base sequence with no known specificity to human genes were purchased from Dharmacon Inc. (Lafayette, Colorado, USA). Transfection was performed as described previously (10). Briefly, 5×103/well of THP-1 cells in 6 well plates were transfected with 425µl growth medium without antibiotics containing 150× dilution of DharmaFECT (Dharmacon Inc.) and 80 nM of siRNA. Seven to 10 days after the transfection, down-regulation of cell surface BAFF was measured using flow cytometry and the levels of mRNA was measured using RT-PCR.
Five micrograms of total RNAs isolated from cells were treated with RNase free DNase (BD-Pharmingen), and then used to generate first-strand cDNAs using the RevertAid™ first strand cDNA synthesis kit with 500 ng oligo (dT)12-18 primers. PCR primers were designed with ABI PRISM Primer Express 2.0 (Applied Biosystems) and made by Geno Tech Corp (Korea). Primer sequences are 5' AGA AGA AGC AGC ACT CTG TC 3' (forward) and 5' CCA TGT GGA GAG AGG TTA AG 3' (reverse) for APRIL, 5' GGT CCA GAA GAA ACA GTC AC 3' (forward) and 5' GGA GTT CAT CTC CTT CTT CC 3' (reverse) for BAFF, and 5' TGG GCT ACA CTG AGC ACC AG 3' (forward) and 5' GGG TGT CGC TGT TGA AGT CA 3'(reverse) for GAPDH. After the PCR reaction, the PCR products were run on 2% agarose gel to confirm the size and purity of the PCR products.
The cells were activated by adding antibodies and fusion proteins to the medium containing 1×106/ml THP-1 cells in RPMI1640 supplemented with 0.1% FBS. The culture supernatants were collected 24 hours after activation for the detection of MMP-9 activity (gelatin zymogram) and the measurement of cytokine concentrations (ELISA) as described previously (11,12).
Carotid endoarterectomy specimens, generously provided by Dr. Jeong-Euy Park, Sungkyunkwan University, School of Medicine, were obtained from patients, aged between 63 to 81, who had undergone surgery at the Samsung Seoul Hospital. RA and osteoarthritis (OA) synovial samples, generously provided by Dr. Eun-Mi Koh, Sungkyunkwan University School of Medicine, were obtained from RA patients during joint replacement therapy. Cases of RA/OA were diagnosed according to the criteria of the American College of Rheumatology. Mononuclear cells were isolated from peripheral blood by density gradient centrifugation using a Histopaque (Sigma-Aldrich, St. Louis, Missouri). Adherent monocytes were isolated after 1 hr incubation and the cells were incubated for 1 week to induce macrophage differentiation (13,14). The purity of the cells (>95% CD14 positive cells) was then confirmed using flow cytometry. These studies were approved by an institutional review committee and the subjects provided informed consent. Specimens were washed with saline and embedded in an optimal cutting temperature medium in order to make frozen sections. Standard 5-µm sections were stained using a LSAB Kit (DAKO, Copenhagen, Denmark), according to instructions provided by the manufacturer.
THP-1 cells expressed high basal levels of both APRIL and BAFF on the cell membrane (9,10). In order to generate cells deficient in BAFF, THP-1 cells were transfected with BAFF-specific siRNA (siBAFF). As shown in Fig. 1A, BAFF expression levels were not detectable in cells transfected with siBAFF while the expression levels of APRIL were not affected. In contrast, cells transfected with control siRNA expressed high levels of both APRIL and BAFF. Accordingly, RT-PCR analysis of siBAFF-transfected cells detected the presence of APRIL mRNA while BAFF mRNA was not detected (Fig. 1B).
Stimulation of either BAFF or APRIL can induce activation signal in THP-1 cells which respond to the stimulation via production of a cytokine, IL-8, or a matrix degrading enzyme, MMP-9 (9,10). The siBAFF-transfected cells were then tested for the responsiveness to BAFF or APRIL-mediated signaling. As shown in Fig. 2A, cells transfected with control siRNA responded to both anti-BAFF and anti-APRIL mAb and expressed high levels of IL-8. Cells transfected with siBAFF failed to respond to the treatment with anti-BAFF mAb but responded to anti-APRIL mAb at a level similar to LPS response. The siBAFF-transfected cells were then treated with agents that mimic its counterparts such as fusion protein containing extracellular domain of TACI (or BCMA) and Fc portion of human immunoglobulin (TACI:Fc or BCMA:Fc). As shown in Fig. 2A, both TACI:Fc and BCMA:Fc stimulated the cells to express IL-8 at a level slightly less than that induced by LPS treatment. Since stimulation of THP-1 cells with BCMA:Fc had not been reported previously, it was analyzed in detail. Treatment of THP-1 cells that are transfected with siBAFF resulted in a dose-dependent expression of both IL-8 and MMP-9 (Fig. 2B). These results, which were performed in the absence of BAFF, clearly demonstrate that stimulation of APRIL with either its counterparts or anti-APRIL mAb leads to the activation of APRIL.
Although APRIL-expressing cells responded to soluble agents such as anti-APRIL mAb, TACI:Fc, or BCMA:Fc, these agents are not generated during normal immune responses or pathologic conditions. It is more likely that membrane-bound form of APRIL will be stimulated through cell-to-cell interaction with cells expressing the counterparts of APRIL. In order to investigate the possibility, B cell lines were used for co-incubation experiment. Both BAFF and APRIL are well known factors required for B cell survival (6-8). Ramos cells, a human B cell line, expressed both TACI and BCMA on the cell surface at high levels but the expression of APRIL was not detected (Fig. 3A). When Ramos cells were co-incubated with siBAFF-transfected THP-1 cells, the expression of both IL-8 and MMP-9 was induced. As expected, pre-incubation of Ramos cells with anti-TACI and anti-BCMA mAb, but not isotype-matching mouse antibody, resulted in a significant reduction of the stimulatory effects (Fig. 3B). This indicates that THP-1 cells can be activated via specific interaction between APRIL and its counterparts during cell-to-cell interaction. The response was not completely blocked by anti-TACI and anti-BCMA suggesting the involvement of other cellular adhesion molecules for the activation of THP-1 cells during the cell-to-cell interaction with Ramos cells.
Detection of APRIL expression in monocytic cell lines and modulation of inflammatory activity through APRIL indicate that APRIL may play an immunoregulatory function during acute and chronic inflammation where macrophages play a major role (15-17). In order to determine whether macrophages express APRIL in vivo, tissue samples from chronic inflammatory diseases were analyzed. CD68 was detected as a specific marker for human macrophages (18). In human atherosclerotic plaques, where macrophages are present in the form of foam cells, a CD68 positive macrophage rich-area was also stained with anti-APRIL mAb (Fig. 4). It is of interest that TACI, which is one of the natural counterparts of APRIL, was also expressed in the same area.
The expression of APRIL and TACI was also tested in synovial tissues from rheumatoid arthritis (RA) and osteroarthritis (OA) patients (Fig. 5A and B). RA tissue samples tended to have more inflammatory cells than OA tissue samples since RA is an autoimmune disease where chronic synovial inflammation destroys the normal architecture of cartilage and bone. Macrophages also play important roles in RA development. The destruction of joint tissue has close correlation with the number of macrophages (19-21) that infiltrate the synovium and the selective depletion of macrophages from the synovial lining prevented both joint inflammation and cartilage destruction (22-24). Macrophage-rich areas in these tissues expressed both APRIL and TACI, albeit the levels were much higher in RA tissues. Interestingly, APRIL and TACI were detected in areas that were not stained for CD68 in RA tissues. This may be due to the expression of APRIL and TACI in cell types other than macrophage or the shedding and subsequent secretion of these cell surface molecules by macrophages to the surroundings. Alternatively, it is also possible that other cell types, such as smooth muscle cells or synovial fibroblasts, may express these molecules. The expression of BCMA was not detected in either RA or OA tissue samples (data not shown).
Expression of APRIL and TACI by macrophage was further confirmed in primary macrophages which are derived from peripheral blood monocytes of RA patients. It is well known that incubation of peripheral blood monocytes for a week induce macrophage differentiation (13,14). In order to detect the expression of APRIL and TACI on the surface of these cells, mAbs and secondary antibodies were added to live primary macrophages before the fixation of the cells. Staining results confirmed that CD68-expressing macrophages express high levels of both APRIL and TACI on the cell surface (Fig. 6).
APRIL and BAFF are members of TNFSF and closely related with each other in their molecular structure and, as a result, share same receptors, TACI and BCMA. Since APRIL and BAFF share the receptors, distinction between APRIL-mediated signaling and BAFF-mediated signaling was difficult. Suppression of BAFF expression by siRNA technique allowed expression of only APRIL. The resulting APRIL positive and BAFF negative cells were then used for so-culture experiment with human B cell line, Ramos that expresses both TACI and BCMA. Current data demonstrate that membrane-bound form of APRIL responds to its counterparts through cell-to-cell interaction.
Previously, the extracellular domain of APRIL has been shown to be expressed on the surface of U937 cells as fusion protein called TWE-PRIL which contains intracellular domain of TWEAK and extracellular domain of ARIIL (25,26). Since the primer used in the experiment cannot distinguish ARPIL and TWE-PRIL, new primer sets that can specifically amplify APRIL, TWEAK, and TWE-PRIL were used to compare normal and siAPRIL transfected THP-1 cells. Results indicated that only APRIL is expressed in THP-1 cells and the expression of TWEAK and TWE-PRIL was not detected. The siAPRIL transfected cells expressed none of them (data not shown). These results indicate that APRIL, not TWE-PRIL, is responsible for the interaction with its counterparts in THP-1 cells and APRIL can generate activation signals.
The expression of both APRIL and its natural counterpart in tissue macrophages raises the possibility that APRIL-mediated regulation of macrophage activity does play a role during immune responses such as inflammation and in pathogenic processes associated with atherosclerosis and/or arthritis. Stimulation of APRIL-expressing macrophages though cell-to-cell interaction with the counterpart-expressing cells may induce inflammatory activation of these cells. In chronically-inflamed, diseased tissue samples, macrophages appear to be the major cell type that express APRIL and its receptor. These molecules, in addition to pro-inflammatory agents which are already known to be expressed by macrophages, will enhance the inflammatory responses and contribute to the pathogenesis of these diseases. Further research is required in animal models and clinical settings in order to identify the importance of APRIL-mediated signaling in the modulation of immune responses and pathogenesis.
Figures and Tables
Figure 1
Transfection of BAFF-specific siRNA resulted in a significant reduction of BAFF expression levels in THP-1 cells. (A) THP-1 cells transfected with control siRNA (Control) or BAFF specific siRNA (siBAFF) were stained with anti-APRIL or anti-BAFF mAb. Histograms from specific staining (open area) and background staining (filled area, stained with mouse IgG1) are compared. (B) RT-PCR analysis was performed in order to detect the presence of ARPIL, BAFF, or GAPDH mRNA in cells transfected with control siRNA (Control) or BAFF specific siRNA (siBAFF). PCR product sizes for APRIL, BAFF, and GAPDH are 394, 370, and 391 bp, respectively. The results are representatives for three independent experiments.

Figure 2
THP-1 cells transfected with BAFF-specific siRNA were stimulated with agents that can interact with APRIL. (A) THP-1 cells transfected with control siRNA (Control) or BAFF specific siRNA (siBAFF) were stimulated with 1µg/ml of LPS, TACI:Fc (TF), BCMA:Fc (BF), human IgG (H), anti-APIRL mAb (A), anti-BAFF mAb (B), or mouse IgG. Culture supernatants were collected 24 hr after the activation and the concentration of IL-8 was measured using double sandwich ELISA. IL-8 concentrations were compared with anti-APRIL stimulated samples which were set to 100%. (B) THP-1 cells transfected with siBAFF was stimulated with 1µg/ml LPS or 1, 3, 10µg/ml of BCMA:Fc for 24 hr. Culture supernatants were then collected for the measurement of IL-8 concentration using ELISA and for the analysis of MMP-9 activity through gelatin zymogram. Data points are represented as mean±SD of triplicate measurements. These experiments were repeated three times with essentially the same results. *p<0.001 when compared with negative control (the first lane). †p<0.001 when compared with the value obtained without T/B pre-incubation.

Figure 3
Ramos cells stimulated THP-1 cells through interaction between APRIL and its counterparts. (A) Ramos cells were stained with mAb against TACI, BCMA, or APRIL. Histograms from specific staining (open area) and background staining (filled area, stained with mouse IgG1) are compared. (B) SiBAFF-transfected THP-1 cells were stimulated with either 1µg/ml of anti-APRIL mAb (A) or co-incubation with Ramos cells. For the blocking, Ramos cells were pre-incubated with 1µg/ml of anti-TACI/anti-BCMA (T/B) mixture or mouse IgG (M) for 20 min. After washing out the antibodies, cells were then incubated with siBAFF-transfected THP-1 cells. Data points are represented as mean±SD of triplicate measurements. These experiments were repeated twice with essentially the same results. *p<0.001 when compared with negative control (the first lane). †p<0.001 when compared with the value obtained without T/B pre-incubation.
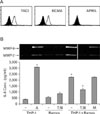
Figure 4
Macrophages in human atherosclerotic plaques express both APRIL and TACI. Consecutive sections of an atherosclerotic plaque were stained with mAbs against CD68 (a marker for macrophages), APRIL, or TACI. Isotype-matching mouse immunoglobulins (mIgG) were used for staining as a negative control. Magnification: ×100. The results are representative for three independent analyses.

Figure 5
Expression of both APRIL and TACI was in macrophages from human arthritic synovial tissues. Consecutive sections of an atherosclerotic plaque RA-synovial tissue (A) or osteoarthritis tissues (B) were stained with mAbs against CD68, APRIL, or TACI. Isotype-matching mouse immunoglobulins (mIgG) were used for staining as a negative control. Magnification: ×100. The results are representative for three independent analyses.
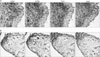
Figure 6
Primary macrophage culture expresses APRIL and TACI on the cell surface. Primary macrophages derived from RA patient peripheral blood monocytes were stained with mAbs against CD68, APRIL, or TACI. In case APPRIL and TACI, specific mAbs were added before fixation and mounting in order to detect the expression of these molecules on the cell surface. Isotype-matching mouse immunoglobulins (mIgG) were used for staining as a negative control. Magnification: ×400. The experiment was repeated twice with essentially the same results.
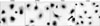
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korean Ministry of Education, Science and Technology (The Regional Core Research Program "Anti-aging and Well-being Research Center" School of Medicine, Kyungpook National University).
References
1. Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998. 188:1185–1190.

2. Kelly K, Manos E, Jensen G, Nadauld L, Jones DA. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000. 60:1021–1027.
3. Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999. 65:680–683.

4. Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003. 21:231–264.

5. Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003. 198:937–945.

6. Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005. 42:763–772.

7. Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006. 5:235–246.

8. Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005. 17:282–289.

9. Jeon ST, Kim WJ, Lee SM, Lee MY, Park SB, Lee SH, Kim IS, Suk K, Choi BK, Choi EM, Kwon BS, Lee WH. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010. 88:148–156.

10. Lee SM, Jeon ST, Kim WJ, Suk KH, Lee WH. Macrophages express membrane bound form of APRIL that can generate immunomodulatory signals. Immunology. 2010. 131:350–356.

11. Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001. 21:2004–2010.

12. Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Netw. 2004. 4:116–122.

13. Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. 1997. 17:265–272.
14. Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1992. 89:10370–10374.

16. Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996. 7:330–335.

17. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.
18. Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993. 81:1607–1613.

19. Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999. 26:717–719.
20. Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996. 39:115–124.

21. Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994. 53:39–44.

22. Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000. 164:4105–4110.

23. van Lent PL, Holthuysen AE, van den Bersselaar LA, van Rooijen N, Joosten LA, van de Loo FA, van de Putte LB, van den Berg WB. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum. 1996. 39:1545–1555.

24. Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB, Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998. 57:408–413.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download