Abstract
CD137 (4-1BB/tnfrsf9) has been shown to co-stimulate T cells. However, agonistic anti-CD137 monoclonal antibody (mAb) treatment can suppress CD4+ T cells, ameliorating autoimmune diseases, whereas it induces activation of CD8+ T cells, resulting in diverse therapeutic activity in cancer, viral infection. To investigate the CD137-mediated T cell suppression mechanism, we examined whether anti-CD137 mAb treatment could affect CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs). Intriguingly, anti-CD137 mAb injection significantly increased CD11b+Gr-1+ cells, peaking at days 5 to 10 and continuing for at least 25 days. Furthermore, this cell population could suppress both CD8+ T cells and CD4+ T cells. Thus, this study demonstrated that, for the first time, anti-CD137 mAb treatment could induce CD11b+Gr-1+ MDSCs under normal conditions, suggesting a possible relationship between myeloid cell induction and CD137-mediated immune suppression.
CD137 (4-1BB/tnfrsf9), a member of tumor necrosis factor (TNF)-receptor family, is a co-stimulatory molecule for T cell activation. Treatment with the agonistic anti-CD137 monoclonal antibody (mAb) in vitro stimulates CD8+ T cells and, to a lesser extent, CD4+ T cells. However, in vivo signaling leads to CD4+ T cell deletion and suppression, although CD8+ T cells are massively expanded and activated (1). This controversy ensures that in vivo agonistic anti-CD137 mAb treatment induces antitumor (2,3) and antiviral immunity (4) through Ag-specific CD8+ T cell activation while suppressing Ag-specific CD4+ T cells and antibody responses (5) that result in the amelioration of autoimmune diseases (6-10) and chronic graft versus host diseases (11).
In a recent study that aimed to clarify the mechanism of this phenomenon, Ag-dependent and CD137-mediated induction of a new cell population, CD11c+CD8+ T cells, was identified in a rheumatoid arthritis model and was shown to be crucial for inducing suppression of Ag-specific CD4+ T cells by interferon gamma (IFNγ) and indoleamine 2,3-dioxygenase (IDO) (9). Furthermore, the emergence of CD11c+ CD8+ T cells by anti-CD137 mAb treatment was also reported in other disease models, such as virus infection, experimental autoimmune uveoretinitis and tumors (12,13). However, the mechanisms responsible for the suppression mediated by anti-CD137 mAb treatment are still unclear. Therefore, to understand the suppressive outcomes of CD137 stimulation, the undefined changes in cell types and functions induced by anti-CD137 mAb treatment need to be further examined.
A recent study showed that interactions between CD137 and CD137L have limited roles in myelopoiesis and the development of dendritic cells (14), suggesting the possibility that CD137 signaling is involved in myeloid cell development and differentiation. Furthermore, it has been shown that in vivo anti-CD137 mAb treatment in a spontaneous lupus-like syndrome model induces the expansion of macrophage/granulocyte population in an IFNγ-dependent manner (6). However, it is still unclear whether anti-CD137 mAb treatment affects the generation of immunosuppressive myeloid cells.
In this study, we investigated the role of CD137 stimulation in the myelopoiesis and immune cell expansion by injecting 300µg of agonistic anti-CD137 mAb (3H3) or rIgG control Ab to naïve BALB/c mice. Five days later, the changes in percentage and number of diverse cell populations, including CD8+ T cells, CD4+ T cells, B cells, NK cells, CD11c+CD8α+ cells and CD11b+Gr-1+ myeloid cells in splenocytes were analyzed using flow cytometry. As a result, anti-CD137 mAb treatment significantly increased both the percentage and real numbers of CD8+ T cells compared to the control Ab treatment (Fig. 1). However, it decreased the percentage of CD4+ T cells, B cells and NK cells, as previously reported (15), but only minimally changed the numbers of cells. As shown in a previous report (9), anti-CD137 mAb treatment increased CD11c+CD8α+ cells and also significantly increased CD11b+Gr-1+ cells both in percentage and real number (p<0.001), suggesting that myelopoiesis of CD11b+Gr-1+ cells could be influenced by CD137 stimulation.
Recent studies suggested that CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) in mice have been shown to accumulate in diverse pathological conditions including cancer, infectious diseases, sepsis, trauma, bone marrow transplantation and some autoimmune diseases, as well as inhibit the function of T cells (16). Therefore, we examined whether increased CD11b+Gr-1+ cells by anti-CD137 mAb treatment could induce characteristics of MDSCs. First, we checked the kinetic changes in CD11b+Gr-1+ cells after anti-CD137 mAb treatment. Single administration of 3H3 led to a significant increase in the CD11b+Gr-1+ cell population of splenocytes 5 days after treatment and continued for at least 25 days (Fig. 2A). The number of CD11b+Gr-1+ cells also increased by the treatment of anti-CD137 mAb and peaked at day 10 (Fig. 2B). Collectively, these data suggested that anti-CD137 mAb treatment could strongly induce the increase in CD11b+Gr-1+ cells.
Next, to investigate that the CD11b+Gr-1+ cells induced by anti-CD137 mAb treatment had suppressive activity similar to MDSCs, the effect of CD137 stimulation-induced CD11b+Gr-1+ cells on Ag-specific T cell proliferation was tested. As a result, OVA Ag-specific proliferation of DO11.10 splenocytes and OT-I splenocytes was down-regulated in a dose-dependent manner by CD11b+Gr-1+ cells isolated at 5 day after anti-CD137 mAb treatment, as compared to CD11b+Gr-1+ cells obtained from naïve mice (Fig. 3A and B). Furthermore, this suppressive activity was also shown in CD11b+Gr-1+ cells isolated at 25 day after anti-CD137 mAb injection (Fig. 3C and D). Collectively, we concluded that CD137 stimulation dramatically induced CD11b+Gr-1+ cells, which suppressed both CD8+ and CD4+ T cells.
In this study, we demonstrated that, for the first time, anti-CD137 mAb treatment significantly induced CD11b+Gr-1+ MDSCs. The presence of this population might explain a novel mechanism of CD137 stimulation-mediated suppression of T cells. Thus, to thoroughly understand the diverse outcome of anti-CD137 mAb treatment, the nature of this population needs to be clarified more precisely.
We measured CD137 expression on naïve CD11b+Gr-1+ cells to investigate that anti-CD137 mAb would act directly on myeloid cells. However, CD137 expression was not observed on CD11b+Gr-1+ cells from spleen and bone marrow (data not shown), suggesting that anti-CD137 mAb treatment might indirectly induce the increase of CD11b+Gr-1+ cells. Likewise, it has been reported that surface expression of CD137 is absent on MDSCs (17). We also checked the IFNγ dependency because IFNγ-dependent, anti-CD137-induced CD11b+Gr-1+ cell expansion was reported in autoimmune disease model (6). However, under normal conditions this phenomenon was not found (data not shown). Thus, it seemed that CD137 stimulation indirectly affected CD11b+ Gr-1+ cells by an IFNγ-independent pathway, and further studies are needed to define the mechanism of anti-CD137 mAb treatment on MDSC induction.
MDSCs can suppress T cell proliferation and activation by secreting iNOS, arginase, reactive oxygen species, nitrating TCR and deprivating cysteine (16). Future studies should investigate whether CD11b+Gr-1+ cells induced by CD137 signaling also use same suppression mechanisms as MDSCs or have a unique system.
Although it was reported that anti-CD137 mAb-injected mice developed a series of immunological anomalies (15), preliminary results from ongoing clinical trials using the humanized anti-CD137 mAb in cancer patients showed that the toxicity profile in these patients was mild (18). However, there is also the possibility that these newly revealed immunosuppressive CD11b+Gr-1+ cells could be observed in anti-CD137 mAb-treated cancer patients and negatively impact immune responses. Thus, it is necessary to confirm whether this cell population could have significant implications in clinical settings.
Figures and Tables
 | Figure 1Agonistic anti-CD137 Ab treatment increases CD11b+Gr-1+ cells in spleen. Naïve BALB/c mice were intraperitoneally (i.p) injected with 300µg of anti-CD137 Ab (3H3) or rIgG control Ab. Five days later, the percentage (A) and number (B) of CD8+ T cells (CD3ε-CD8α+), CD4+ T cells (CD3ε-CD4+), B cells (B220+CD3ε-), NK cells (CD49b+CD3ε-), CD11c+CD8α+ cells and CD11b+Gr-1+ cells in splenocytes were examined (n=3~4/group). *p<0.05, **p<0.005, †p<0.001. Data are representative of at least three separate experiments. |
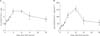 | Figure 2The kinetics of splenic CD11b+Gr-1+ cells due to anti-CD137 Ab treatment. The kinetic changes in percentage (A) and number (B) of splenic CD11b+Gr-1+ cells were measured after 300µg of anti-CD137 Ab i.p injection. |
 | Figure 3Increased xCD11b+Gr-1+ cells by CD137 stimulation suppress T cell proliferation. Total splenocytes (4×105) of DO11.10 mice (A, C) or OT-I mice (B, D) were stimulated with OVA protein (A, C: 100µg/ml, B, D: 250µg/ml) and serially diluted naïve splenic CD11b+Gr-1+ cells or 3H3 induced CD11b+Gr-1+ cells (A, B: 5 days after 3H3 treatment; C, D: 25 days after 3H3 treatment) were added. Three days after culture, T cell proliferation was examined including an 18 hr pulse with 4µCi/ml [3H] thymidine. |
ACKNOWLEDGEMENTS
We thank Dr. R. Mittler of Emory University (Atlanta, GA) for providing 3H3 hybridoma.
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-E00203).
References
1. Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006. 84:726–736.

2. Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, Lynch DH. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002. 169:1792–1800.

3. Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004. 109:499–506.

4. Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002. 3:536–541.

5. Hong HJ, Lee JW, Park SS, Kang YJ, Chang SY, Kim KM, Kim JO, Murthy KK, Payne JS, Yoon SK, Park MJ, Kim IC, Kim JG, Kang CY. A humanized anti--4-1BB monoclonal antibody suppresses antigen-induced humoral immune response in nonhuman primates. J Immunother. 2000. 23:613–621.

6. Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002. 8:1405–1413.

7. Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002. 168:1457–1465.

8. Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi N, Hoffmann MK, Mittler RS. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003. 111:1505–1518.

9. Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004. 10:1088–1094.

10. Foell JL, Diez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS, Hoffmann MK, Mittler RS, Dannecker GE. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004. 113:89–98.

11. Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, Kwon BS, Kwon B. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005. 105:2206–2213.

12. Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS. 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol. 2005. 238:76–86.

13. Vinay DS, Kim CH, Choi BK, Kwon BS. Origins and functional basis of regulatory CD11c+CD8+ T cells. Eur J Immunol. 2009. 39:1552–1563.
14. Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol. 2008. 9:917–926.

15. Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L, Vella AT, Mittler RS. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007. 178:4194–4213.

16. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009. 9:162–174.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download