Abstract
Purpose
Several studies have shown that viral respiratory infections induce more severe respiratory symptoms in atopic patients than in normal subjects. We attempted to investigate if there is any difference in the viral etiology, clinical manifestations, production of interleukin (IL)-8, and regulated on activation in normal T-cell expressed and secreted (RANTES) between atopic and non-atopic subjects with lower respiratory infections.
Methods
Sera and nasopharyngeal aspirates (NPA) were collected from 97 children with lower respiratory infections who were admitted to the pediatric ward. Seventy-one children were classified as atopic subjects. We detected respiratory viruses with multiplex reverse transcriptase-polymerase chain reaction in NPA and measured total immunoglobulin E (IgE) and specific IgE in sera. IL-8 and RANTES levels measured in sera by enzyme-linked immunosorbent assay, etiology, and clinical manifestations were compared between atopic and non-atopic subjects. Atopic patients were defined as having elevated specific IgE to more than one allergen or age-matched, high serum total IgE levels.
Results
Both serum IL-8 and RANTES levels were significantly higher in atopic than in non-atopic patients. There was no significant difference in viral etiology and clinical diagnosis between the two groups. The frequency of wheezing was higher in atopic than in non-atopic patients.
Conclusion
Our study showed that both serum IL-8 and RANTES levels and the frequency of wheezing were significantly higher in atopic than in non-atopic patients. That suggests that chemokine responses to viral respiratory infection may be different between atopic and non-atopic patients and may be associated with a difference in clinical manifestation, such as wheezing, between the two groups. However, further prospective large-scaled studies are required to clarify our conclusion.
Go to : 
References
1. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986; 108(5 Pt 1):635–46.

2. Jung BS, Oh JS, Cho B, Kim HH, Lee JS. A clinical study of respiratory tract infections in hospitalized children. Pediatr Allergy Respir Dis. 1996; 6:60–73.
3. Acute respiratory infections in under-fives: 15 million deaths a year. Lancet. 1985; 2:699–701.
5. Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002; 324:763.

6. Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TA, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003; 111:1008–16.

7. Gern JE, Calhoun W, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997; 155:1872–6.

8. Psarras S, Volonaki E, Skevaki CL, Xatzipsalti M, Bossios A, Pratsinis H, et al. Vascular endothelial growth factor-mediated induction of angiogenesis by human rhinoviruses. J Allergy Clin Immunol. 2006; 117:291–7.

9. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005; 201:937–47.

10. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002; 57:328–32.

11. Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003; 168:1091–4.
12. Holgate ST, Lemanske RF, O'Byrne PM, Kaku-manu S, Busse WW. Asthma pathogenesis. Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. editors.Middleton's Allergy: Principles and Practice. 7th Ed.St. Louis: Mosby;2009. p. 893–919.

13. Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994; 15:127–33.

14. Yousefi S, Hemmann S, Weber M, Hölzer C, Hartung K, Blaser K, et al. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol. 1995; 154:5481–90.
15. Teran LM, Davies DE. The chemokines: their potential role in allergic inflammation. Clin Exp Allergy. 1996; 26:1005–19.

16. Smith PH, Ownby DR. Clinical significance of immunoglobulin E. Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. editors.Middleton's Allergy: Principles and Practice. 7th Ed.St. Louis: Mosby;2009. p. 845–57.
17. Saarinen UM, Juntunen K, Kajosaari M, Bj 2009én F. Serum immunoglobulin E in atopic and nonatopic children aged 6 months to 5 years. A follow-up study. Acta Paediatr Scand. 1982; 71:489–94.
18. Gern JE, Martin MS, Anklam KA, Shen K, Ro-berg KA, Carlson-Dakes KT, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002; 13:386–93.

19. Grünberg K, Timmers MC, Smits HH, de Klerk EP, Dick EC, Spaan WJ, et al. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997; 27:36–45.

20. Turner RB, Weingand KW, Yeh CH, Leedy DW. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998; 26:840–6.

21. Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000; 162:2226–31.

22. Chung HL, Kim HH, Lee EH, Kim SG. Eosinophil activation induced by RANTES (regulated upon activation, normal T-cell-expressed -secreted) in respiratory syncytial virus bronchiolitis. Pediatr Allergy Respir Dis. 1999; 9:192–9.
23. Sung RY, Hui SH, Wong CK, Lam CW, Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr. 2001; 160:117–22.

24. Yoon JS, Lee MH, Lee JS. Serum ECP, RANTES and eotaxin levels in infants with bronchiolitis. Korean J Pediatr. 2004; 47:170–6.
25. Bardin PG, Sanderson G, Robinson BS, Holgate ST, Tyrrell DA. Experimental rhinovirus infection in volunteers. Eur Respir J. 1996; 9:2250–5.

26. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and nonasthmatic individuals: a longitudinal cohort study. Lancet. 2002; 359:831–4.

27. Yang BS, Kim SY, Lee GH, Lee JH, Choi EJ, Kim JK, et al. Relationship between atopic status and immunoregulatory cytokines in respiratory syncytial virus bronchiolitis. Pediatr Allergy Respir Dis. 2004; 14:30–7.
28. Chung HL, Kim WT, Kim JK, Choi EJ, Lee JH, Lee GH, et al. Relationship between atopic status and nasal interleukin 10 and 11 levels in infants with respiratory syncytial virus bronchiolitis. Ann Allergy Asthma Immunol. 2005; 94:267–72.

29. van Benten IJ, van Drunen CM, Koevoet JL, Koopman LP, Hop WC, Osterhaus AD, et al. Reduced nasal IL-10 and enhanced TNFalpha responses during rhinovirus and RSV-induced upper respiratory tract infection in atopic and nonatopic infants. J Med Virol. 2005; 75:348–57.
Go to : 
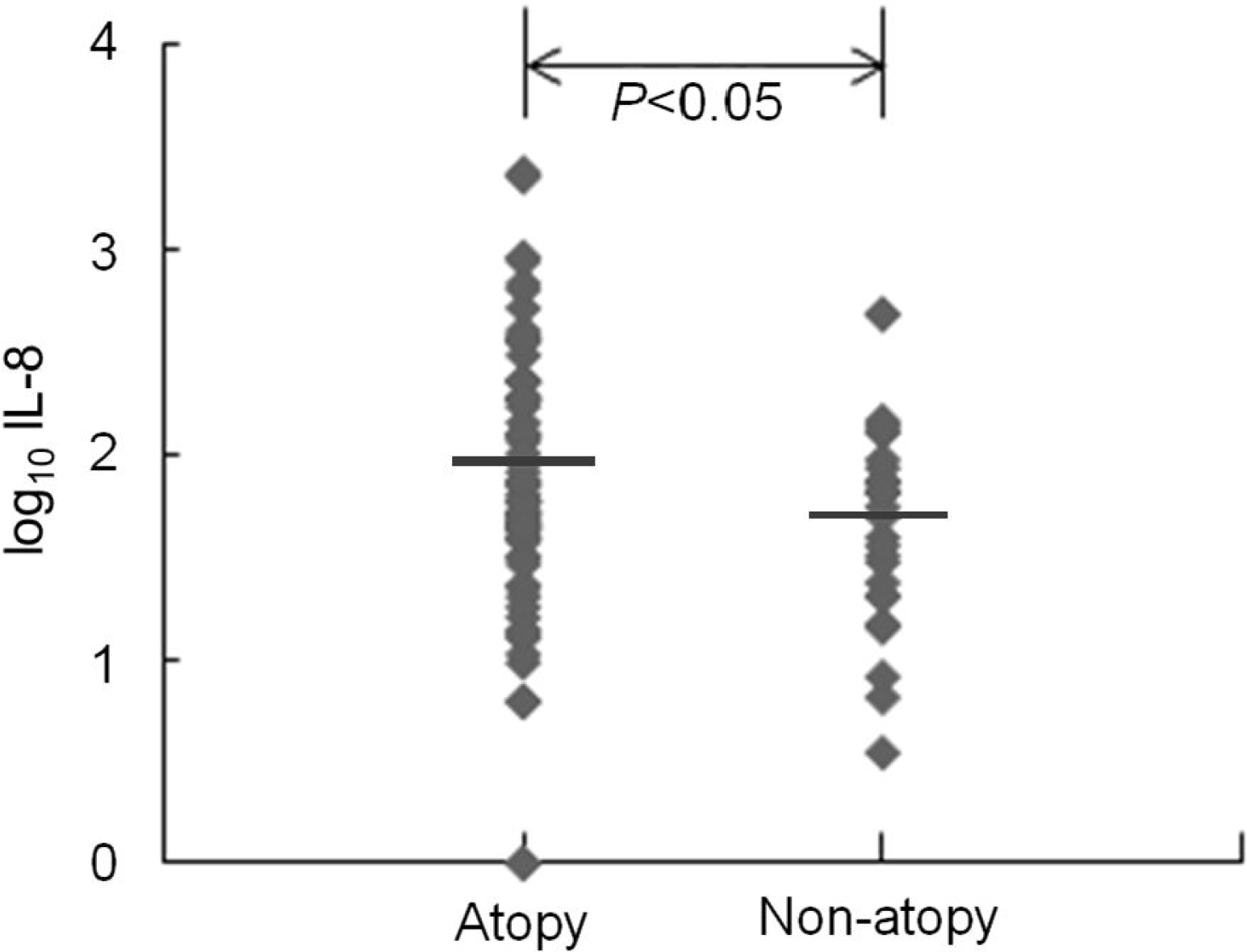 | Fig. 1.Serum interleukin (IL)-8 levels in atopy group were significantly higher than those in non-atopy group during lower respiratory infection. Horizontal bars represent geometric mean. |
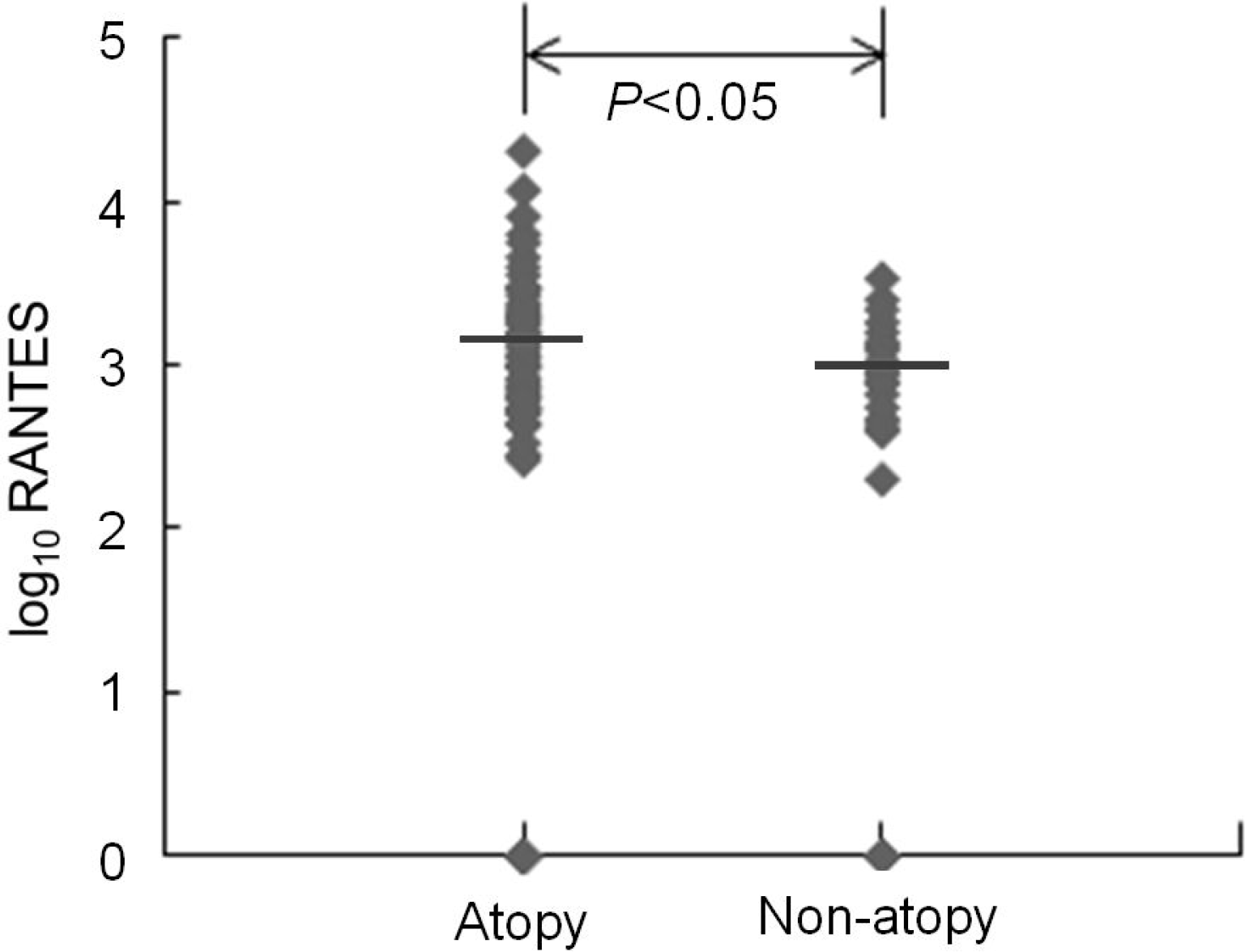 | Fig. 2.Serum regulated on activation in normal T-cell expressed and secreted (RANTES) levels in atopy group were significantly higher than those in non-atopy group during lower respiratory infection. Horizontal bars represent geometric mean. |
Table 1.
Characteristics of Atopic and Non-Atopic Patients
| Variable | Atopy | Non-atopy |
|---|---|---|
| No. of Subjects | 72∗ | 28 |
| Age (mo)† | 35∗ | 29 |
| Sex (M:F) | 50:22∗ | 17:9 |
| Hospital day, day† (range) | 6.0 (2–12)∗ | 5.8 (3–10) |
Table 2.
The Comparison of Isolated Respiratory Viruses from the Children with Lower Respiratory Tract Infections According to Atopic Status




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download