Abstract
Purpose
The objective of this study was to identify differences in the clinical manifestations and allergic indices between monosensitized and polysensitized children.
Methods
We reviewed retrospective data from the medical records of patients who had chronic or recurrent respiratory symptoms and visited the pediatric clinic at Chung-Ang University Hospital for an evaluation of allergic diseases from January 2003 to January 2011. The patients were categorized into nonsensitized (n=111), monosensitized (n=149), and polysensitized (n=205) groups according to skin prick tests (as classified by five allergen groups). We compared gender, age, family history, admission history, food sensitization, total immunoglobulin E (IgE), peripheral eosinophil counts, eosinophil cationic protein (ECP) levels, forced expiratory volume in 1 second (FEV1), and methacholine provocation tests among the three groups.
Results
The frequency of food sensitivity was highest in the polysensitized group (n=101, 49.3%), followed by the monosensitized (n=8, 5.4%) and nonsensitized groups (n=0) (P<0.001). The FEV1 was significantly lower in the polysensitized group than that in the monosensitized and nonsensitized groups (79.4 20.2% vs. 87.2 16.0% vs. 87.6 17.1%, respectively) (± ± ± P=0.013). The total IgE and ECP levels were significantly higher in the polysensitized patients than those in the other patients (P<0.001 and <0.001, respectively). Differences in gender, age, peripheral eosinophil count, and bronchial hyper-responsiveness were not identified between the monosensitized and polysensitized groups.
References
1. Bousquet J. Pro: Immunotherapy is clinically indicated in the management of allergic asthma. Am J Respir Crit Care Med. 2001; 164:2139–40.

2. Dean T, Venter C, Pereira B, Arshad SH, Grundy J, Clayton CB, et al. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J Allergy Clin Immunol. 2007; 120:1166–71.

4. Kim KW, Kim EA, Kwon BC, Kim ES, Song TW, Sohn MH, et al. Comparison of allergic indices in monosensitized and polysensitized patients with childhood asthma. J Korean Med Sci. 2006; 21:1012–6.

5. Bousquet J, Becker WM, Hejjaoui A, Chanal I, Lebel B, Dhivert H, et al. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple-pollen species. II. Efficacy of a double-blind, placebocontrolled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991; 88:43–53.
6. Kang H, Yu J, Yoo Y, Kim DK, Koh YY. Coincidence of atopy profile in terms of monosensiti-zation and polysensitization in children and their parents. Allergy. 2005; 60:1029–33.

7. Ownby DR. Environmental factors versus genetic determinants of childhood inhalant allergies. J Allergy Clin Immunol. 1990; 86(3 Pt 1):279–87.

8. Prigione I, Morandi F, Tosca MA, Silvestri M, Pistoia V, Ciprandi G, et al. Interferon-gamma and IL-10 may protect from allergic polysensitization in children: preliminary evidence. Allergy. 2010; 65:740–2.

9. Roberts G, Peckitt C, Northstone K, Strachan D, Lack G, Henderson J, et al. Relationship between aeroallergen and food allergen sensitization in childhood. Clin Exp Allergy. 2005; 35:933–40.

10. de Jong AB, Dikkeschei LD, Brand PL. Sensitization patterns to food and inhalant allergens in childhood: a comparison of non-sensitized, monosensitized, and polysensitized children. Pediatr Allergy Immunol. 2011; 22:166–71.
12. Horigome K, Bullock ED, Johnson EM Jr. Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J Biol Chem. 1994; 269:2695–702.

13. Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein–a clue to the function of the eosinophil granulocyte. Respir Res. 2011; 12:10.

14. Kim KW, Lee KE, Kim ES, Song TW, Sohn MH, Kim KE. Serum eosinophil-derived neurotoxin (EDN) in diagnosis and evaluation of severity and bronchial hyperresponsiveness in childhood asthma. Lung. 2007; 185:97–103.

15. Ghunaim N, Wickman M, Almqvist C, Söderst-röm L, Ahlstedt S, van Hage M. Sensitization to different pollens and allergic disease in 4-year-old Swedish children. Clin Exp Allergy. 2006; 36:722–7.

16. Ciprandi G, Cirillo I, Tosca MA, Vizzaccaro A. Bronchial hyperreactivity and spirometric impairment in polysensitized patients with allergic rhinitis. Clin Mol Allergy. 2004; 2:3.
17. Johnston SL, Clough JB, Pattemore PK, Smith S, Holgate ST. Longitudinal changes in skin-prick test reactivity over 2 years in a population of schoolchildren with respiratory symptoms. Clin Exp Allergy. 1992; 22:948–57.

18. Kuehr J, Karmaus W, Frischer T, Hendel-Kramer A, Weiss K, Moseler M, et al. Longitudinal variability of skin prick test results. Clin Exp Allergy. 1992; 22:839–44.

19. Peat JK, Salome CM, Woolcock AJ. Longitudinal changes in atopy during a 4-year period: relation to bronchial hyperresponsiveness and respiratory symptoms in a population sample of Australian schoolchildren. J Allergy Clin Immunol. 1990; 85(1 Pt 1):65–74.

Fig. 1.
The comparison of (A) eosinophil count, (B) eosinophil fraction, (C) log (total immunoglobulin E) and (D) eosinophilc cationic protein (ECP) levels in the three groups. ∗P<0.05.
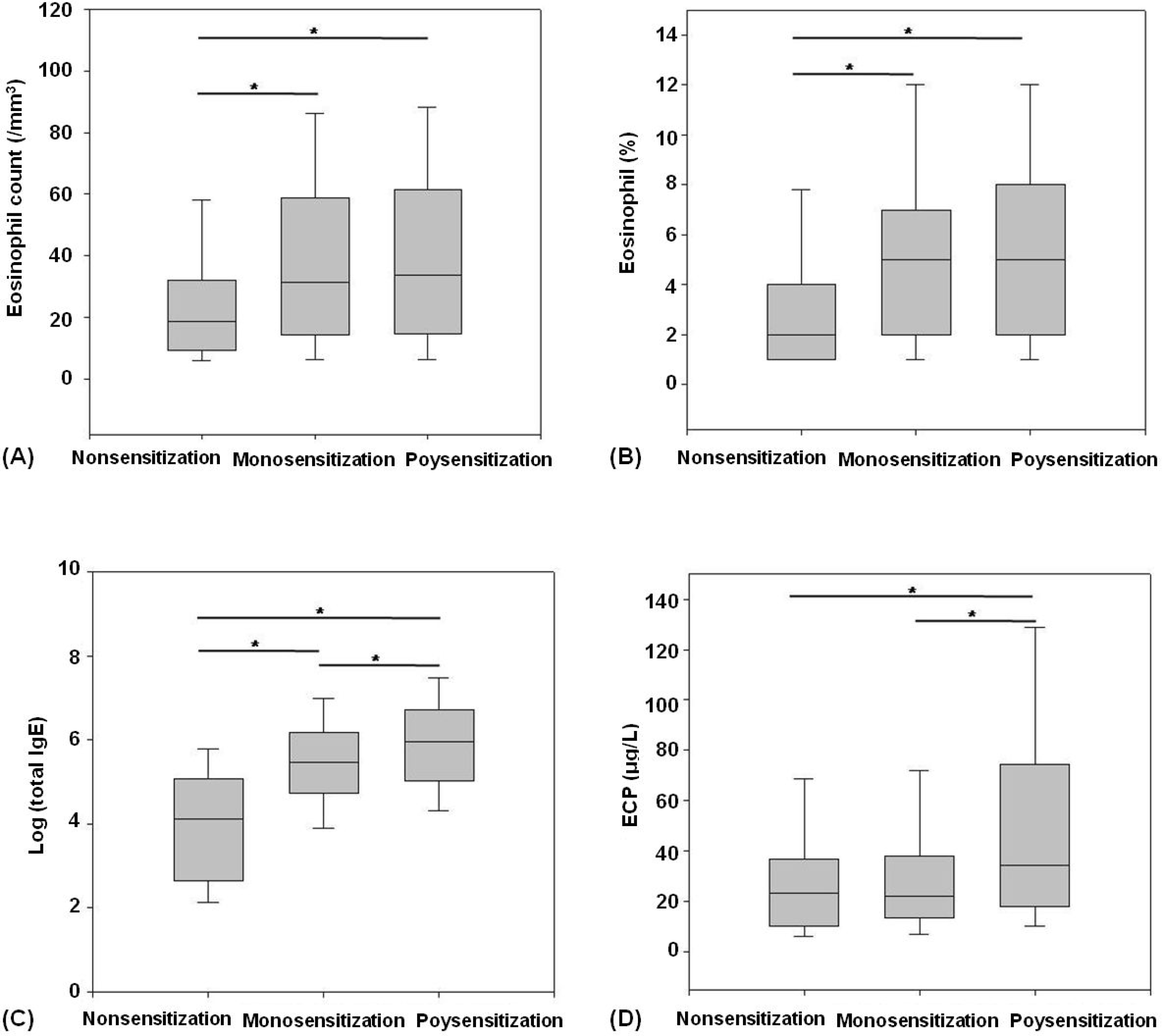
Fig. 2.
Comparison of forced expiratory volume in 1 second in nonsensitized, monosensitized, and polysensitized groups. ∗P <0.05.
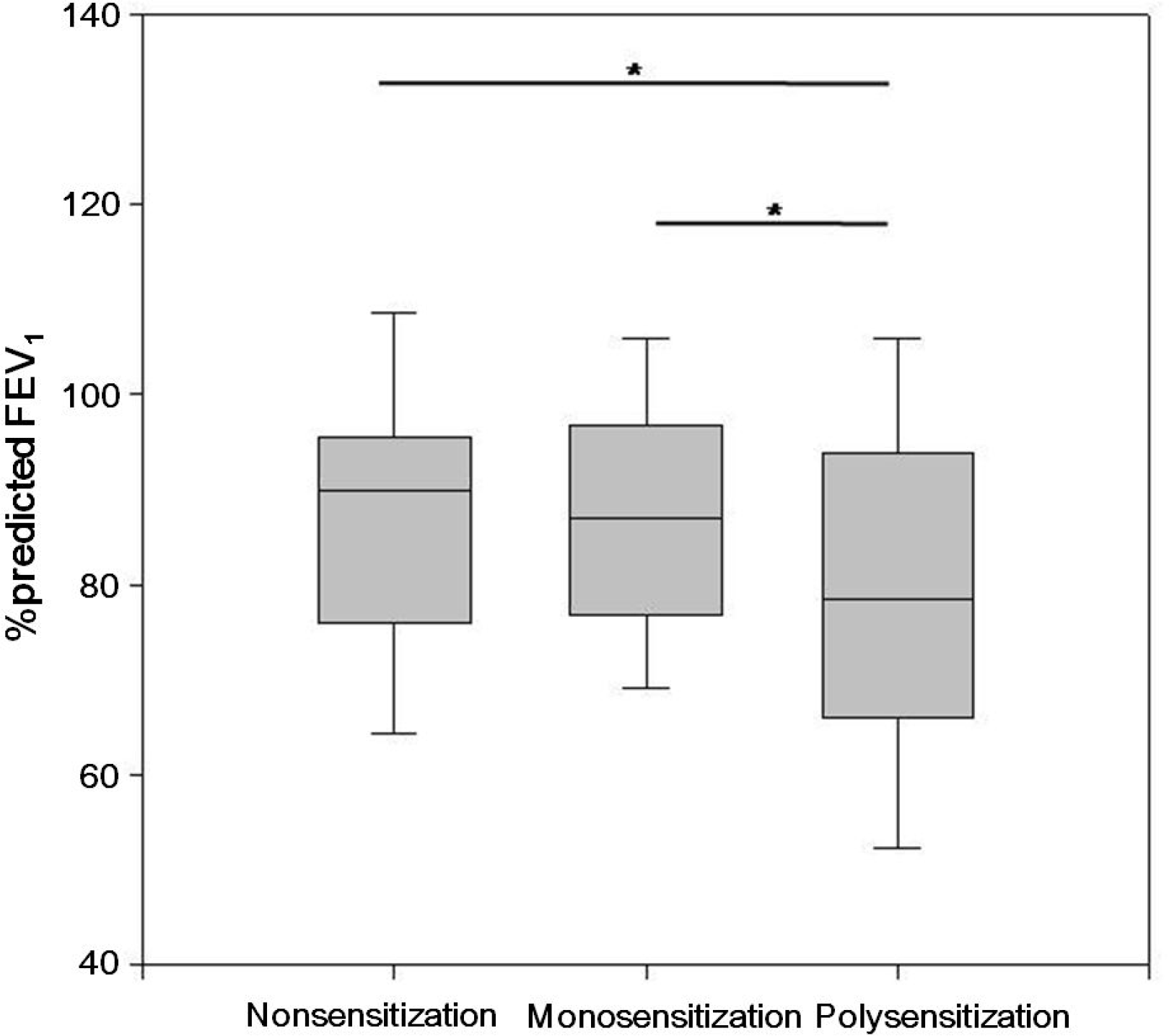
Table 1.
Clinical Characteristics and History of the Study Population
| Nonsensitized children (n=111) | Monosensitized children (n=149) | Polysensitized children (n=205) | P-value | |
|---|---|---|---|---|
| Sex (M/F) | 62/49 | 96/53 | 131/74 | 0.306 |
| Age (yr)∗ | 7.9 3.5 ± | 8.5 3.1 ± | 8.5 3.5 ± | 0.225 |
| Allergic familial history | 23 (20.7%) | 39 (26.2%) | 89 (26.8%) | 0.462 |
| No. of hospitalization∗ | 1.2 2.3 ± | 0.8 1.3 ± | 1.1 2.0 ± | 0.242 |
| Food sensitization | 0 | 8 (5.4%) | 101 (49.3%) | <0.001 |
| BHR | 26 (65.0%) | 46 (80.7%) | 39 (73.6) | 0.221 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download