Abstract
Purpose
Walnut (WN) allergy in young children has rarely been reported in Asia. This study focused on the clinical characteristics of WN allergy, co-sensitization, and cross-reactivity between WN and peanut (PN) in young Korean children.
Methods
This study was based on a data analysis of 22 patients, all under the age of 4 years, who were diagnosed with allergic disease at Ajou University Hospital from January 2009 to December 2010. They were suspected to have a WN allergy or needed a screening examination to exclude food allergy. Sera from all children were analyzed for PN-, WN-, and pine nut-specific immunoglobulin E (IgE) (ImmunoCAP). Clinical details, feeding, and familial history of patients were collected by medical history. Additionally, we produced WN, PN, and pine nut extracts, and sera were tested with an enzyme linked immunosorbentassay inhibition test.
Results
The subjects were 16 male and 6 female with a median aged of 24 months. Ten of 22 had a definite history of WN exposure. Among them, two (4.28 kU/L, 11.1 kU/L) were diagnosed with anaphylaxis, four (7.34 to 27.4 kU/L) were diagnosed with angioedema, and four (1.35 to 3.17 kU/L) were diagnosed with urticaria. We confirmed that PN in the IgE-ELISA was profoundly inhibited by the WN extract.
Go to : 
References
2. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011; 128:e9–17.

3. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004; 19:716–23.

4. Sicherer SH, Muñoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999; 103:559–62.

5. Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001; 107:191–3.

6. Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992; 327:380–4.

7. Sicherer SH, Furlong TJ, Muñoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001; 108:128–32.

8. Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998; 102:e6.

9. Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007; 7:175–81.

10. Muñoz-Furlong A, Weiss CC. Characteristics of food-allergic patients placing them at risk for a fatal anaphylactic episode. Curr Allergy Asthma Rep. 2009; 9:57–63.

11. Rajan TV. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003; 24:376–9.

12. Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ. 1996; 313:518–21.

13. Ewan PW. Clinical study of peanut and nut allergy in 62 consecutive patients: new features and associations. BMJ. 1996; 312:1074–8.

14. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001; 357:111–5.

15. Viquez OM, Summer CG, Dodo HW. Isolation and molecular characterization of the first genomic clone of a major peanut allergen, Ara h 2. J Allergy Clin Immunol. 2001; 107:713–7.

16. Viquez OM, Konan KN, Dodo HW. Structure and organization of the genomic clone of a major peanut allergen gene, Ara h 1. Mol Immunol. 2003; 40:565–71.

17. Dodo HW, Viquez OM, Maleki SJ, Konan KN. cDNA clone of a putative peanut (Arachis hypo-gaea L.) trypsin inhibitor has homology with peanut allergens Ara h 3 and Ara h 4. J Agric Food Chem. 2004; 52:1404–9.

18. Teuber SS, Jarvis KC, Dandekar AM, Peterson WR, Ansari AA. Identification and cloning of a complementary DNA encoding a vicilin-like proprotein, jug r 2, from english walnut kernel (Juglans regia), a major food allergen. J Allergy Clin Immunol. 1999; 104:1311–20.

19. Lauer I, Foetisch K, Kolarich D, Ballmer-Weber BK, Conti A, Altmann F, et al. Hazelnut (Corylus avellana) vicilin Cor a 11: molecular characterization of a glycoprotein and its allergenic activity. Biochem J. 2004; 383(Pt 2):327–34.

20. Wang F, Robotham JM, Teuber SS, Tawde P, Sathe SK, Roux KH. Ana o 1, a cashew (Anacardium occidental) allergen of the vicilin seed storage protein family. J Allergy Clin Immunol. 2002; 110:160–6.

22. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–6.

23. Lee KI, Kim SY. The table of food supply and demand in 2001. Reston: Korea Rural Economic Institute;2002. ;275.
24. Hwnag YJ. The table of food supply and demand in 2007. Reston: Korea Rural Economic Institute;2008. ;287.
26. Sicherer SH, Wood RA, Stablein D, Lindblad R, Burks AW, Liu AH, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010; 126:1191–7.

27. O'Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010; 126:574–80.e1.
28. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009; 124(3 Suppl 2):R7–12.

29. Radauer C, Breiteneder H. Evolutionary biology of plant food allergens. J Allergy Clin Immunol. 2007; 120:518–25.

30. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005; 116:1087–93.

31. Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008; 122:145–51.

32. Hourihane JO, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy. 1997; 27:634–9.

33. Yu JW, Kagan R, Verreault N, Nicolas N, Joseph L, St Pierre Y, et al. Accidental ingestions in children with peanut allergy. J Allergy Clin Immunol. 2006; 118:466–72.

34. Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr. 2000; 137:749–55.

35. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000; 106(2 Pt 1):346–9.
Go to : 
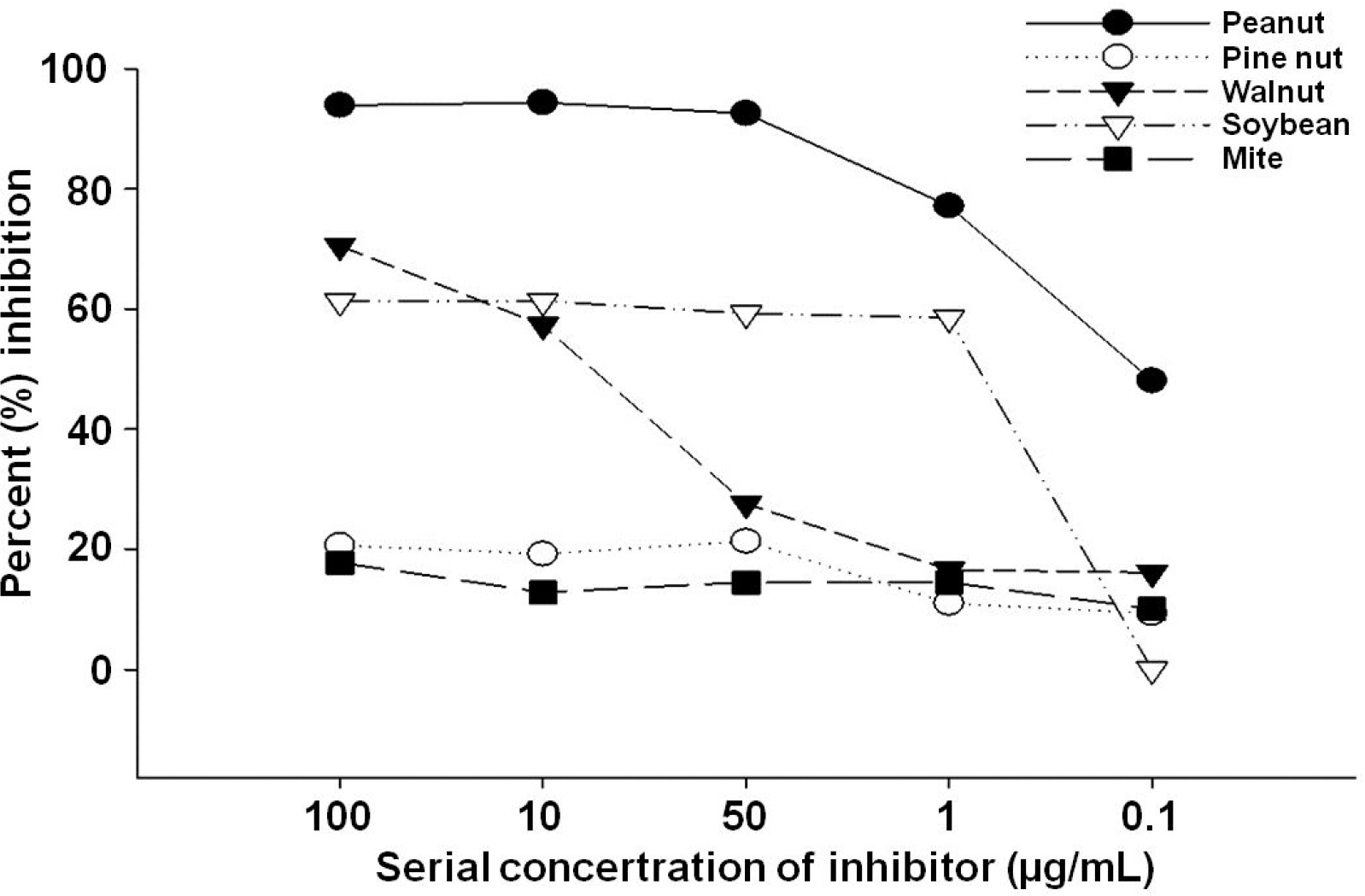 | Fig. 1.Peanut immunoglobulin E-enzyme linked immunosorbentassay inhibition test using inhibitors; crude extract of peanut, walnut, pine nut, soybean and house dust mite. |
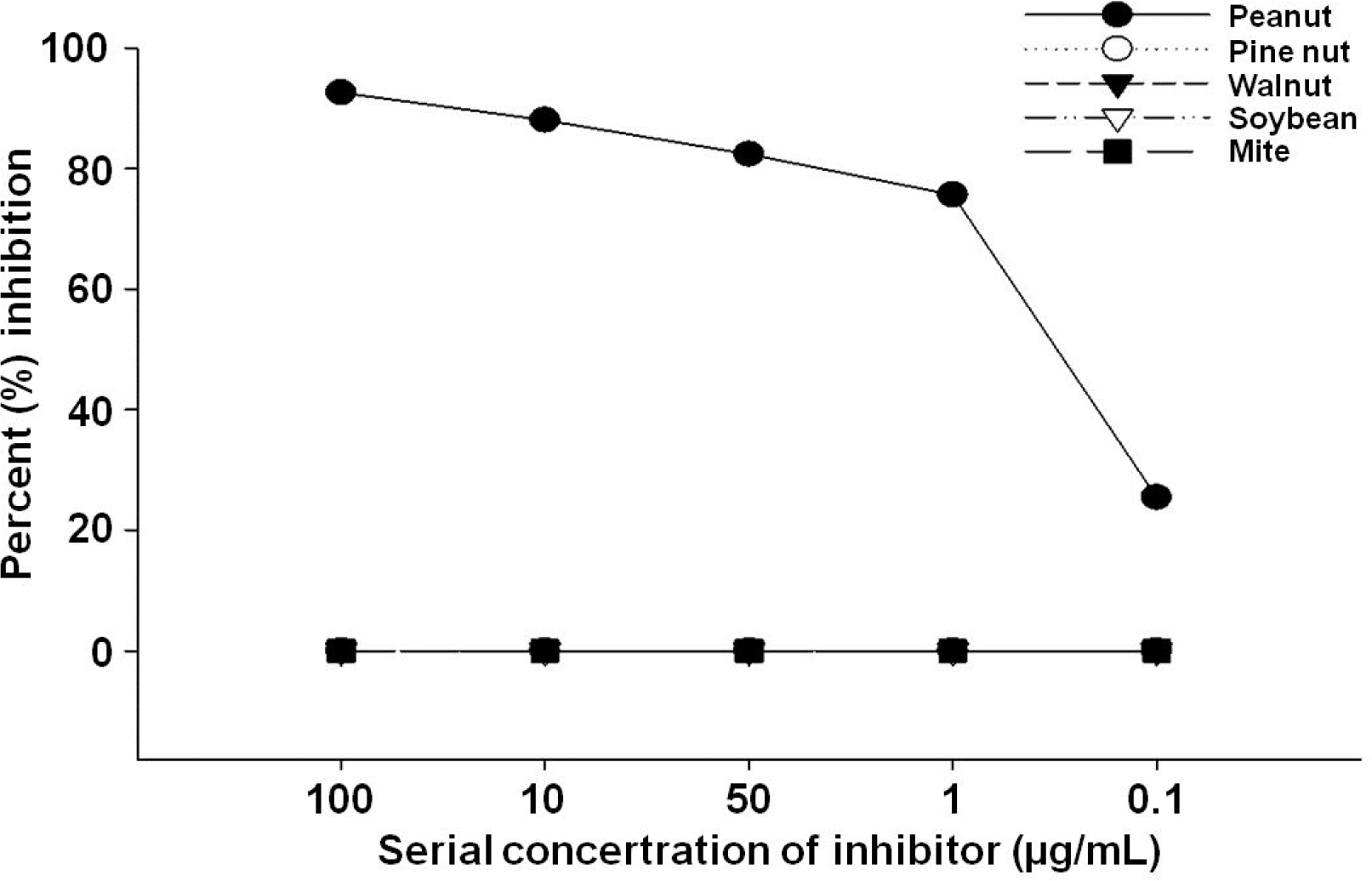 | Fig. 2.Walnut immunoglobulin E-enzyme linked immunosorbentassay inhibition test using inhibitors; crude extract of peanut, walnut, pine nut, soybean and house dust mite. |
Table 1.
Clinical Characteristics in Walnut Sensitized Subjects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download