Abstract
Purpose
Fractional exhaled nitric oxide (FeNO) has been proposed as a tool for assessing airway inflammation in patients with atopic asthma. We evaluated the relationship between FeNO with asthma control test (ACT) scores and spirometry values in children with atopic asthma.
Methods
One hundred twenty-six children with atopic asthma, 8–16 years of age, were included in the study. None of the participants received regular controller therapy for at least 4 weeks before the study. The ACT (for children >12 years of age) or the Childhood Asthma Control Test (C-ACT; for children between the ages of 8 and 11 years of age), FeNO measurements and pulmonary function tests were performed.
Results
The geometric mean of the FeNO in children with atopic asthma (16.1 parts per billion [ppb]; 95% CI, 14.5–17.8 ppb) was significantly higher than that healthy controls (7.5 ppb; 95% CI, 7.0–8.1 ppb; P<0.001). ACT or C-ACT scores were >20 in 82% of enrolled patients and correlated with the %FEV1, FEV1/FVC, and %FEF25–75. However, FeNO was not related to %FEV1, FEV1/FVC, %FEF25–75, and scores for asthma controls. FeNO levels in asthmatic children were not significantly different with respect to age, gender, BMI, and tobacco exposure.
Go to : 
References
1. Crimi E, Spanevello A, Neri M, Ind W, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998; 157:4–9.

2. Gibson PJ. Monitoring the patient with asthma: an evidencebased approach. J Allergy Clin Immunol. 2000; 106:17–26.

3. Lundback B, Dahl R. Assessment of asthma control and its impact on optimal treatment strategy. Allergy. 2007; 62:611–9.

4. Green RH, Brightling CE, McKenna S, Harga-don B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomized controlled trial. Lancet. 2002; 360:1715–21.
5. Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005; 172:831–6.
6. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005; 352:2163–73.

7. Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007; 176:231–7.
8. Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomized controlled trial. Lancet. 2008; 372:1065–72.
9. Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between ex-haled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001; 164:1376–81.

10. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998; 53:91–5.

11. Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001; 164:738–43.

12. Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax. 2002; 57:889–96.

13. Jones SL, Herbison P, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR. et al. Exhaled NO and assessment of antiinflammatory effects of inhaled steroid: dose response relationship. Eur Respir J. 2002; 20:601–8.
14. Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003; 21:433–8.

15. Ko HS, Chung SH, Choi YS, Choi SH, Rha YH. Relationship between exhaled nitric oxide and pulmonary function test in children with asthma. Korean J Pediatr. 2008; 51:181–87.

16. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.
17. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007; 119:817–25.

18. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999; 159:179–87.

19. American Thoracic society. Recommendation for standardized procedure for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. Am J Respir Crit Care Med. 1999; 160:2104–17.
20. Sivan Y, Gadish T, Fireman E, Soferman R. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr. 2009; 155:211–6.

21. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–6.

22. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Available at:. http://www.ginasthma.com/Guidelineitem.
23. Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006; 117:549–56.

24. Piacentini GL, Peroni DG, Bodini A, Bonafiglia E, Rigotti E, Baraldi E, et al. Childhood Asthma Control Test and airway inflammation evaluation in asthmatic children. Allergy. 2009; 64:1753–7.

25. van Den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000; 162:953–7.

26. Leung TF, Li CY, Lam CW, Au CS, Yung E, Chan IH, et al. The relation between obesity and asthmatic airway inflammation. Pediatr Allergy Immunol. 2004; 15:344–50.

27. Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005; 116:1163–4.

28. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999; 159:69–73.

29. Latzin P, Griese M. Exhaled hydrogen peroxi-de, nitrite and nitric oxide in healthy children: decrease of hydrogen peroxide by atmospheric nitric oxide. Eur J Med Res. 2002; 7:353–8.
30. Warke TJ, Mairs V, Fitch PS, Ennis M, Shields MD. Possible association between passive smoking and lower exhaled nitric oxide in asthmatic children. Arch Environ Health. 2003; 58:613–6.

31. Dinakar C, Lapuente M, Barnes C, Garg U. Real -life environmental tobacco exposure does not affect exhaled nitric oxide levels in asthmatic children. J Asthma. 2005; 42:113–8.
Go to : 
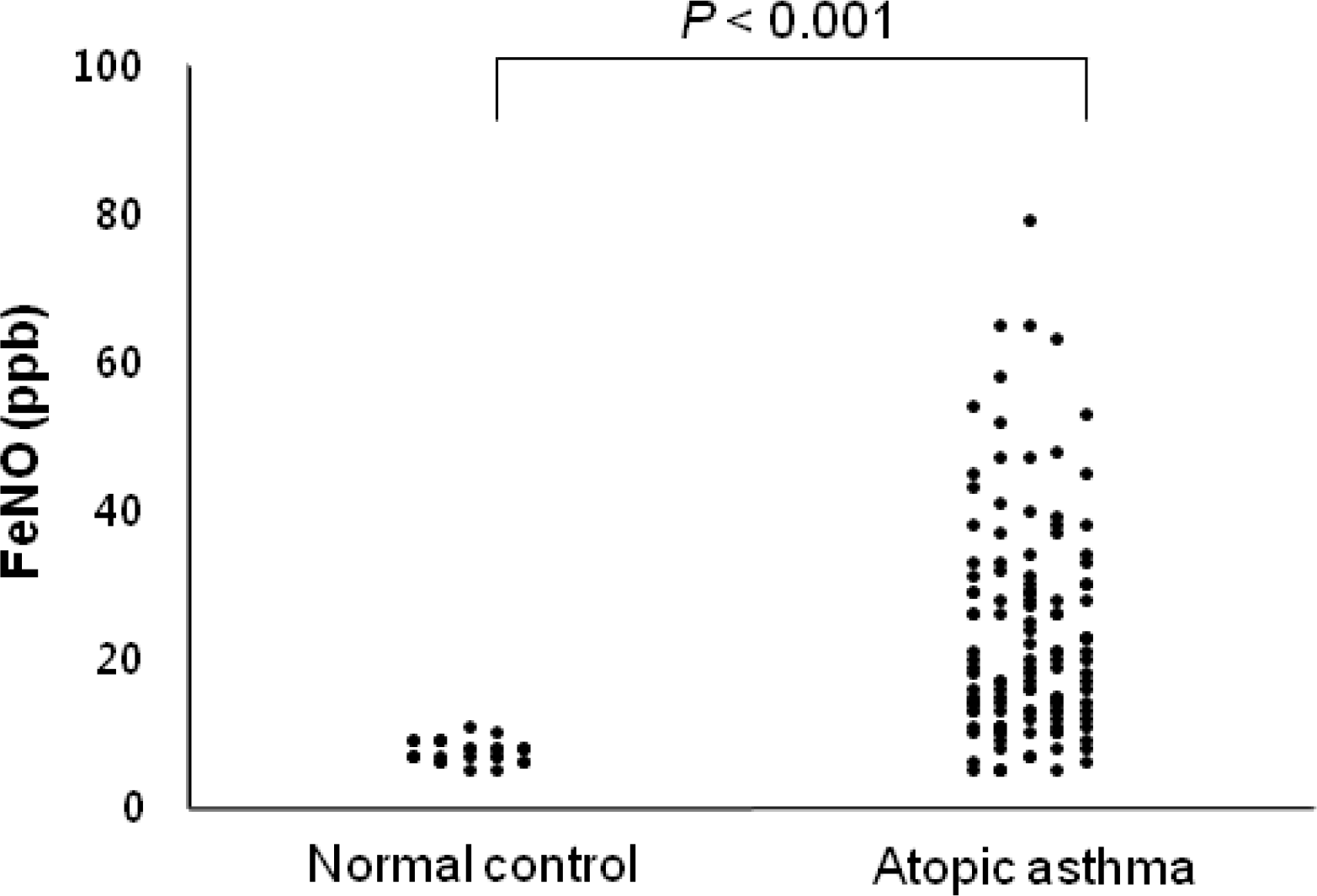 | Fig. 1.Comparison of FeNO between patients with atopic asthma and non-atopic healthy controls. |
Table 1.
Characteristics of the Patients
Table 2.
Correlation of C-ACT Score (A) and ACT Score (B) with Spirometric Values and FeNO in Children with Atopic Asthma
Table 3.
Correlation of FeNO with Spirometric Values, Age, and BMI in Children with Atopic Asthma
| Correlation coefficient | P value | |
|---|---|---|
| FEV1% predicted | –0.091 | 0.294 |
| FEV1/FVC | 0.051 | 0.294 |
| FVC% predicted | –0.129 | 0.150 |
| FEF25–75% predicted | –0.020 | 0.827 |
| Age (years) | 0.103 | 0.251 |
| BMI z-score | –0.087 | 0.335 |
Table 4.
Relevance of FeNO to Gender and Tobacco Exposure
| FeNO∗ ppb (95%CI) | P value | ||
|---|---|---|---|
| Gender | Male | 19.8 | 0.451 |
| (17.7–22.3) | |||
| Female | 17.8 | ||
| (13.6–23.2) | |||
| Tobacco exposure | Yes | 20.8 | 0.055 |
| (18.0–24.0) | |||
| No | 16.1 | ||
| (14.1–19.6) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download