Abstract
Purpose
The aim of this study was to compare the effects of breast milk (BM) feeding with those of maternal cow milk (CM) restriction and extensively hydrolyzed CM formula feeding on the duration of CM allergy as well as changes in specific immunoglobulin E (IgE) levels in infants with CM allergy.
Methods
Children diagnosed with CM allergy before 12 months age and BM fed were included retrospectively. CM allergy was diagnosed by CM specific IgE over 0.35 kU/L and 1) obvious clinical symptoms, 2) a suspicious history with positive provocation test, or 3) CM specific IgE over the 95% positive predictive value and subsequent documented report of clinical symptoms. The patients were classified into three groups by feeding regimen: BM group, extensively hydrolyzed formula (eHF) group, or mixed feeding (MF) group. Analysis of the groups regarding the duration of food allergy and changes in CM specific IgE was then performed.
Results
Forty-six children were included. Twenty-four children were in the BM group, 13 children were in the eHF group, and 9 children comprised the MF group. Thirteen patients reached tolerance. The means of the tolerance age were 69.7±5.4 months in the BM group, 36.6±4.6 months in the eHF group, and 38.2±7.9 months in the MF group. The survival curves of tolerance showed significant difference among the three groups (P=0.04). CM specific IgE levels measured at a second time period were 9.6 kU/L (interquartile range, 3.6–44.2) in the BM group, 2.0 kU/L (1.0–18.0) in the eHF group, and 4.8 kU/L (0.2–10.4) in the MF group (P=0.04).
Go to : 
References
3. Saarinen KM, Juntunen-Backman K, Järvenpää AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow's milk allergy: a prospective study of 6209 infants. J Allergy Clin Immunol. 1999; 104(2 Pt 1):457–61.

4. Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006; 117(2 Suppl Mini-Primer):): S470–5.

5. García-Ara C, Boyano-Martínez T, Díaz-Pena JM, Martín-Muñoz F, Reche-Frutos M, Martín -Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows' milk protein in the infant. J Allergy Clin Immunol. 2001; 107:185–90.

7. Host A. Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol. 1994; 5(5 Suppl):1–36.

8. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. JAllergy Clin Immunol. 2007; 120:1172–7.

9. Santos A, Dias A, Pinheiro JA. Predictive factors for the persistence of cow's milk allergy. Pediatr Allergy Immunol. 2010; 21:1127–34.

10. Levy Y, Segal N, Garty B, Danon YL. Lessons from the clinical course of IgE-mediated cow milk allergy in Israel. Pediatr Allergy Immunol. 2007; 18:589–93.

11. Sampaio G, Marinho S, Prates S, Morais-Almeida M, Rosado-Pinto J. Transient vs persistent cow's milk allergy and development of other allergic diseases. Allergy. 2005; 60:411–2.
12. Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010; 303:1848–56.
13. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000; 106(2 Pt 1):346–9.
14. Muraro A, Dreborg S, Halken S, H⊘st A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part II. Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatr Allergy Immunol. 2004; 15:196–205.

15. Muraro A, Dreborg S, Halken S, H⊘st A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part III: critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol. 2004; 15:291–307.

16. Casas R, Böttcher MF, Duchén K, Björkstén B. Detection of IgA antibodies to cat, beta-lactoglobulin, and ovalbumin allergens in human milk. J Allergy Clin Immunol. 2000; 105(6 Pt 1):1236–40.
17. Kalliomäki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-beta in breast milk: a potential regulator of atopic disease at an early age. J Allergy Clin Immunol. 1999; 104:1251–7.
18. Pérez-Machado MA, Ashwood P, Thomson MA, Latcham F, Sim R, Walker-Smith JA, et al. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur J Immunol. 2003; 33:2307–15.
19. Hoppu U, Kalliomäki M, Laiho K, Isolauri E. Breast milk–immunomodulatory signals against allergic diseases. Allergy. 2001; 56(Suppl 67):23–6.
20. Terracciano L, Bouygue GR, Sarratud T, Veglia F, Martelli A, Fiocchi A. Impact of dietary regimen on the duration of cow's milk allergy: a random allocation study. Clin Exp Allergy. 2010; 40:637–42.

21. Pastorello EA, Stocchi L, Pravettoni V, Bigi A, Schilke ML, Incorvaia C, et al. Role of the elimination diet in adults with food allergy. J Allergy Clin Immunol. 1989; 84(4 Pt 1):475–83.

22. Kim JS, Sicherer S. Should avoidance of foods be strict in prevention and treatment of food allergy? Curr Opin Allergy Clin Immunol. 2010; 10:252–7.

23. Allen CW, Kemp AS, Campbell DE. Dietary advice, dietary adherence and the acquisition of tolerance in egg-allergic children: a 5-yr followup. Pediatr Allergy Immunol. 2009; 20:213–8.

24. Schulmeister U, Swoboda I, Quirce S, de la Hoz B, Ollert M, Pauli G, et al. Sensitization to human milk. Clin Exp Allergy. 2008; 38:60–8.

25. Bernard H, Negroni L, Chatel JM, Clement G, Adel-Patient K, Peltre G, et al. Molecular basis of IgE cross-reactivity between human beta-casein and bovine beta-casein, a major allergen of milk. Mol Immunol. 2000; 37:161–7.
26. Kuitunen M, Savilahti E, Sarnesto A. Human alpha-lactalbumin and bovine beta-lactoglobulin absorption in infants. Allergy. 1994; 49:354–60.
27. Friedman NJ, Zeiger RS. The role of breastfeeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005; 115:1238–48.

28. Osterlund P, Smedberg T, Hakulinen A, Heikkilä H, Järvinen KM. Eosinophil cationic protein in human milk is associated with development of cow's milk allergy and atopic eczema in breastfed infants. Pediatr Res. 2004; 55:296–301.

29. Shek LP, Soderstrom L, Ahlstedt S, Beyer K, Sampson HA. Determination of food specific IgE levels over time can predict the development of tolerance in cow's milk and hen's egg allergy. J Allergy Clin Immunol. 2004; 114:387–91.

Go to : 
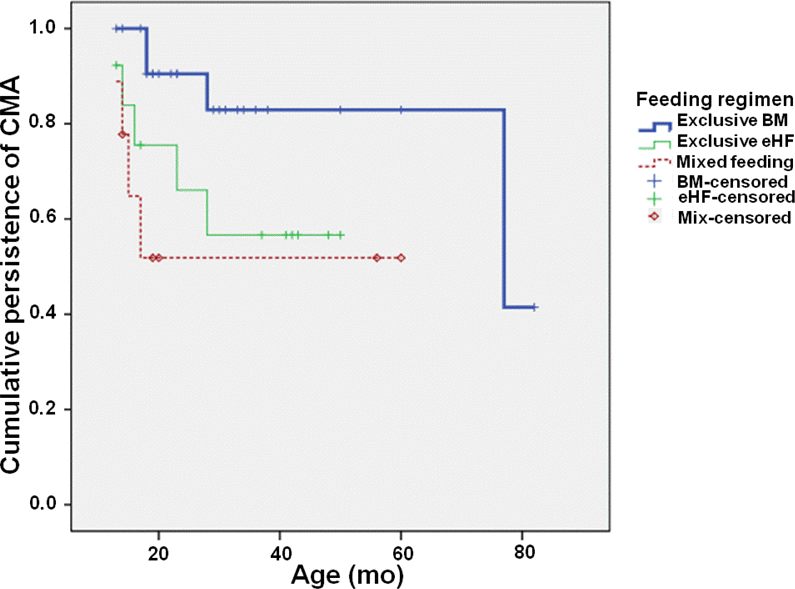 | Fig. 2.Comparison of survival curve for persistence between breast milk, extensively hydrolyzed formula and mixed feeding groups. BM, breast milk; eHF, extensively hydrolyzed formula. |
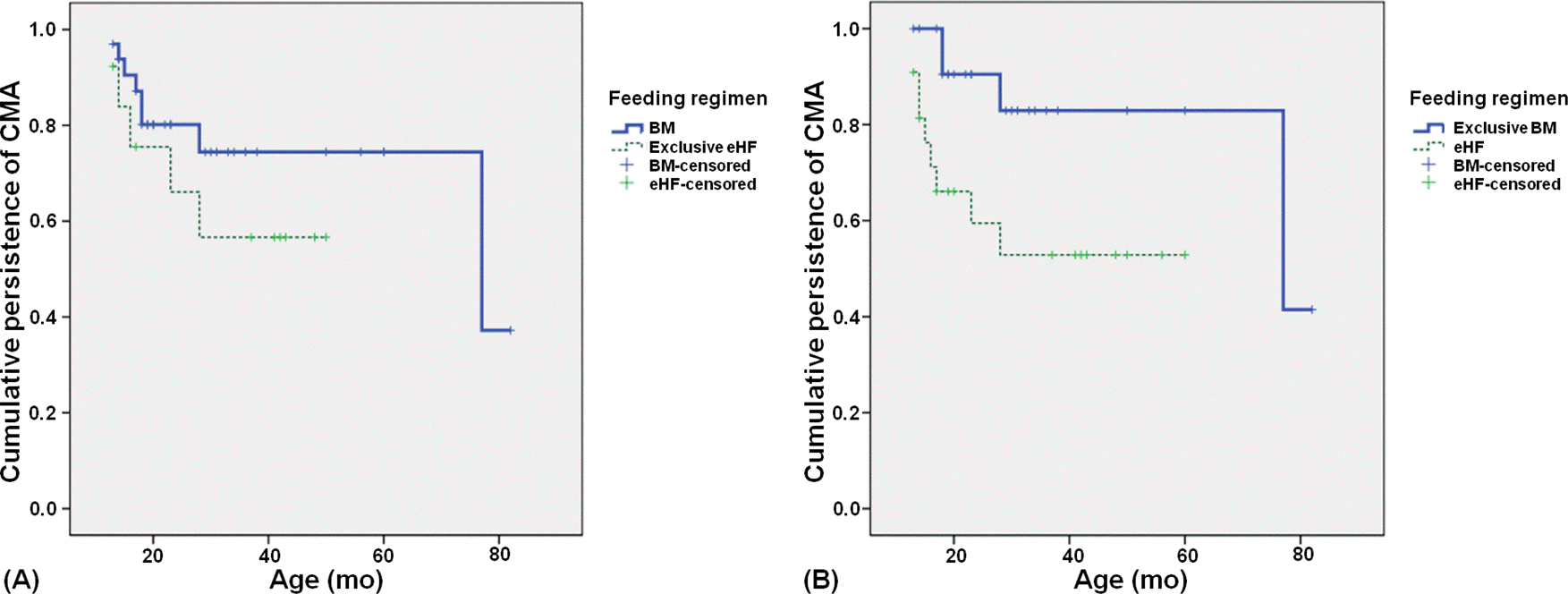 | Fig. 3.Comparison of survival curve for persistence between (A) exclusive hydrolyzed formula (eHF) group and the other feeding groups (P =0.284), (B) exclusive breast milk group and the other feeding groups (P =0.020). BM, breast milk. |
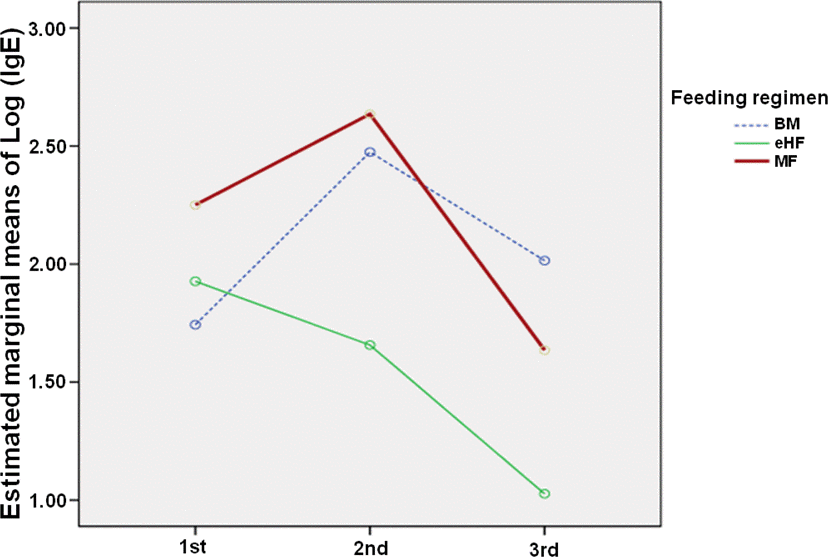 | Fig. 4.The changes of specific immunoglobulin E (IgE) in each feeding groups. The levels of specific IgE are log transformed to show changes of means in each groups. BM, breast milk; eHF, extensively hydrolyzed formula; MF, mixed feeding. |
Table 1.
Comparison Among Breast Milk Group, Mixed Feeding Gruop and Extensively Hydrolyzed Formula Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download