Abstract
Purpose
The measurement of exhaled nitric oxide (eNO) is a noticeable tool that reflects asthmatic airway inflammation. However, the eNO values might be variable according to the patient's condition and the method of measurement. The aim of this study was to compare the values of eNO measured by two different eNO analyzers in asthmatic children (Niox mino® [Aerocrine; Solna, Sweden] and CLD88® [Eco Medics; Durten, Switzerland].
Methods
One hundred four asthmatic children and 59 healthy controls were enrolled. The study participants underwent pulmonary function testing before and after inhaled bronchodilator treatment, a methacholine provocation test, and sputum induction; the eNO concentration was then measured.
Results
The value of eNO measured by Niox mino® was significantly lower than the value of eNO measured by CLD88® (30.7±25.0 vs. 38.6±29.2 ppb, P<0.001). The intraclass correlation coefficient was 0.786 (P<0.001). The eNO concentration was significantly increased in asthmatic children than controls (38.4±27.9 vs. 17.2±9.0 ppb, P<0.001 by Niox mino®; 47.8±31.8 vs. 22.2± 12.7 ppb, P<0.001 by CLD88®). The eNO concentration was significantly correlated with the FEV1/ FVC (r=-0.382, P<0.001 [Niox mino®]; r=-0.273, P=0.001 [CLD88®], percent sputum eosinophils (r=0.257, P=0.032 [Niox mino®]; r=0.297, P=0.017 [CLD88®]), and PC20 (r=-0.333, P <0.001 [by Niox mino®] r=-0.240, P=0.003 [CLD88®]).
Go to : 
References
1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Revised 2006 NIH publication. Available at. http://www.ginasthma.com. [accessed March 2008].
2. Busse WW, Pedersen S, Pauwels RA, Tan WC, Chen YZ, Lamm CJ, et al. The inhaled steroid treatment as regular therapy in early asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2008; 121:1167–74.

3. Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF Jr., Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004; 170:426–32.
4. Yang EG, Kim WJ, Kwon BC, Choi SY, Sohn MH, Kim KE. Relationship among pulmonary function, bronchial hyperresponsiveness, and atopy in children with clinically stable asthma. Lung. 2006; 184:73–9.

5. Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997; 52:498–501.

6. Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004; 84:731–65.

7. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998; 53:91–5.

8. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005; 352:2163–73.

9. Boot JD, Ridder L, de Kam ML, Calderon C, Mascelli MA, Diamant Z. Comparison of exhaled nitric oxide measurements between NIOX MINO electrochemical and Ecomedics chemiluminescence analyzer. Respir Med. 2008; 102:1667–71.

10. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. This Joint Statement of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) was adopted by the ATS Board of Directors, December 2004, and by the ERS Executive Committee, June 2004 Am J Respir Crit Care Med. 2005; 171:912–30.
11. Yoshikawa T, Shoji S, Fujii T, Kanazawa H, Kudoh S, Hirata K, et al. Severity of exercise-induced bronchoconstriction is related to airway eosinophilic inflammation in patients with asthma. Eur Respir J. 1998; 12:879–84.

12. Korn S, Telke I, Kornmann O, Buhl R. Measurement of exhaled nitric oxide: comparison of different analysers. Respirology. 2010; 15:1203–8.

13. Alving K, Janson C, Nordvall L. Performance of a new handheld device for exhaled nitric oxide measurement in adults and children. Respir Res. 2006; 7:67.

14. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongse J, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–6.

15. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999; 159:69–73.

16. Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J.
17. Tsang KW, Ip SK, Leung R, Tipoe GL, Chan SL, Shum IH, et al. Exhaled nitric oxide: the effects of age, gender and body size. Lung. 2001; 179:83–91.

18. Muller KC, Jorres RA, Magnussen H, Holz O. Comparison of exhaled nitric oxide analyzers. Respir Med. 2005; 99:631–7.
19. Pizzimenti S, Bugiani M, Piccioni P, Heffler E, Carosso A, Guida G, et al. Exhaled nitric oxide measurements: correction equation to compare handheld device to stationary analyzer. Respir Med. 2008; 102:1272–5.

20. Choi BS, Jee HM, Park YH, Kim KW, Sohn MH, Kim KE. Relationship between exhaled nitric oxide concentration and pulmonary function/airway hyperresponsiveness in asthmatic children. Pediatr Allergy Respir Dis. 2009; 19:291–9.
21. Ko HS, Chung SH, Choi YS, Choi SH, Rha YH. Relationship between exhaled nitric oxide and pulmonary function test in children with asthma. Korean J Pediatr. 2008; 51:181–7.

22. Nah KM, Park Y, Kang EK, Kang H, Koh YY, Lee SW, et al. Exhaled nitric oxide concentration in children with asthma and allergic rhinitis: Association with atopy and bronchial hyperresponsiveness. J Korean Pediatr Soc. 2003; 46:284–90.
23. Spallarossa D, Battistini E, Silvestri M, Sabatini F, Fregonese L, Brazzola G, et al. Steroid-naïve adolescents with mild intermittent allergic asthma have airway responsiveness and elevated exhaled nitric oxide levels. J Asthma. 2003; 40:301–10.
24. Piacentini GL, Bodini A, Costella S. Exhaled nitric oxide and sputum eosinophil markers of inflammation in asthmatic children. Eur Respir J. 1999; 13:1386–90.

25. Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000; 16:1008–15.
Go to : 
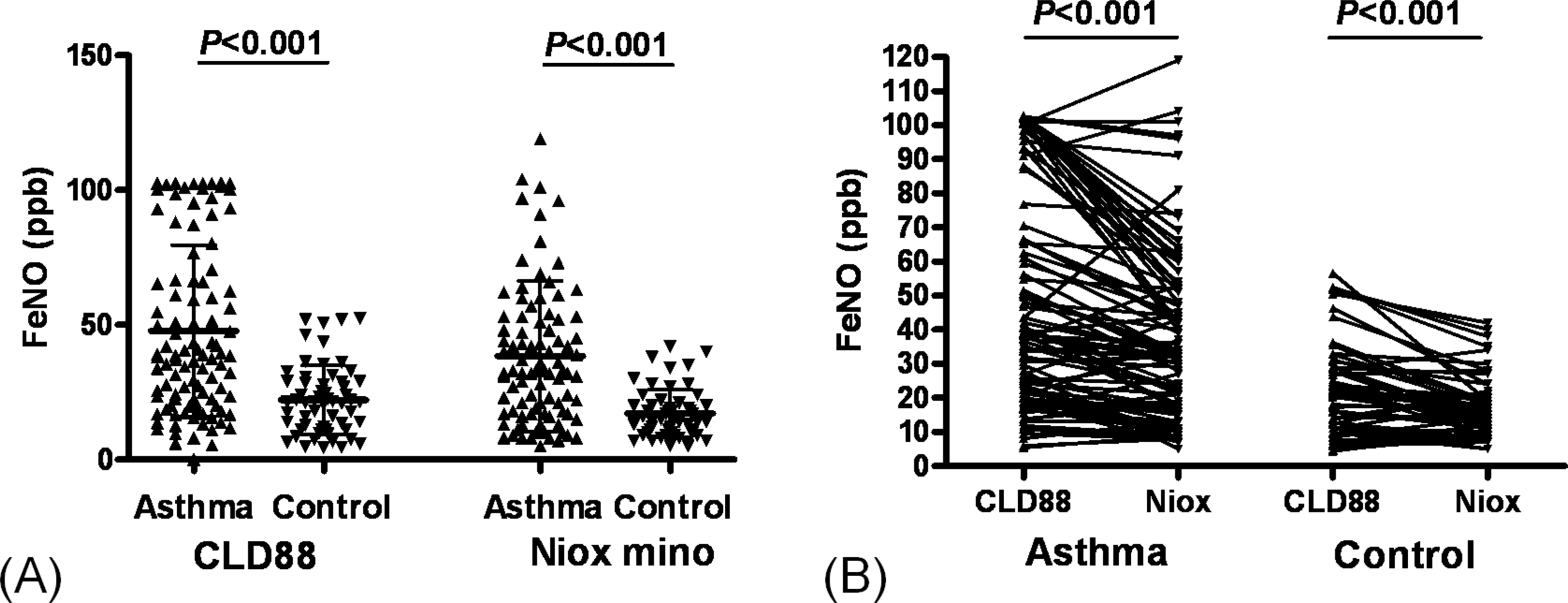 | Fig. 1.Comparison of exhaled Nitric Oxide level. A. Comparison of exhaled Nitric Oxide (eNO) values between Asthma and Control group. Asthma group had higher eNO values, which were measured by CLD88® (P <0.001) and Niox mino® (P <0.001), than control. The horizontal lines show mean and standard deviation. B. Comparison of eNO values between by CLD88® and Niox mino®. Exhaled NO levels by CLD88® (P <0.001) were higher than those by Niox mino® (P <0.001) in both groups. |
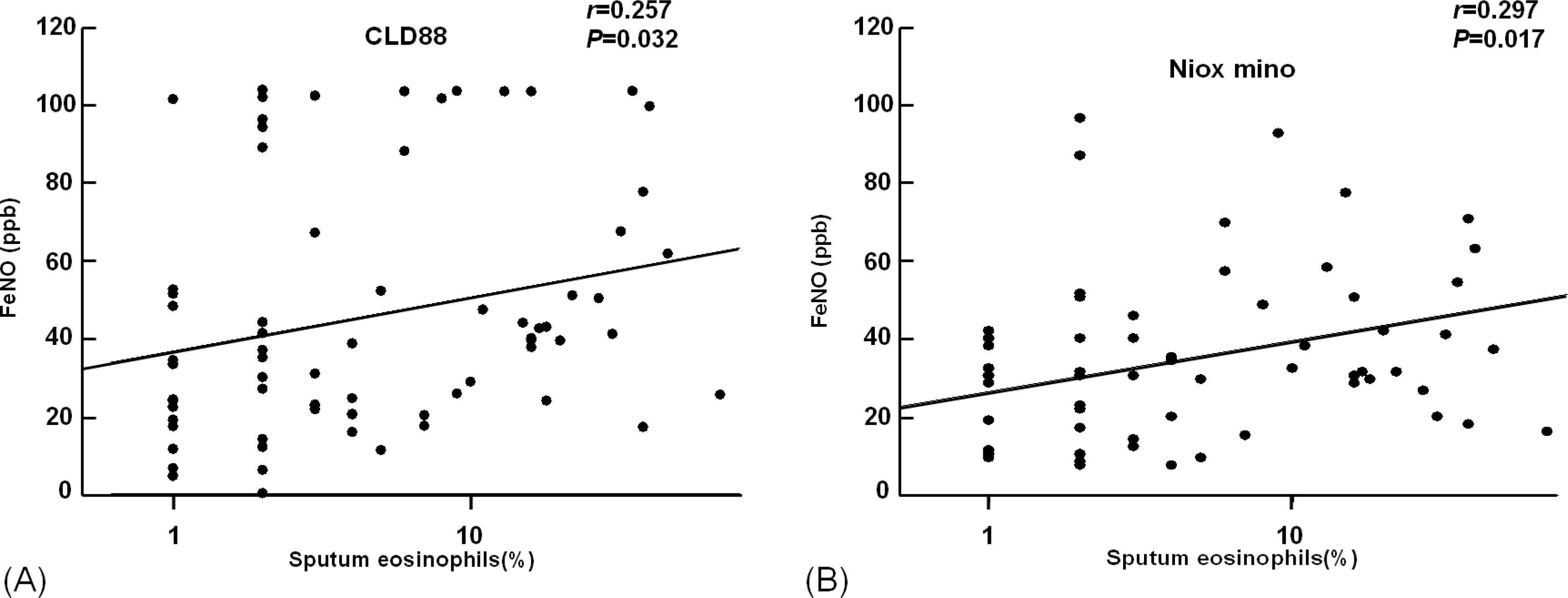 | Fig. 2.Correlation between eNO and Sputum Eosinophils (%) A. by CLD88®, B. by Niox mino®. Exhaled NO values were correlated with sputum eosinophils (%) (r =0.257, P =0.032, by CLD88®; r = 0.297, P=0.017, by Niox mino®) |
Table 1
Demographics and Pulmonary Functions of Subjects
| Asthma | Control | |
| No. of Subjects | 104 | 59 |
| Sex, M/F | 71/33 | 36/23 |
| Age, yr | 10.2±2.8 | 11.0±2.8 |
| Height, cm | 139.9±15.5 | 144.6±14.3 |
| Weight, kg | 38.4±13.9 | 41.9±13.0 |
| BMI | 19.0±3.2 | 19.6±3.2 |
| FEV1, %pred | 94.6±17.5∗ | 102.5±14.8 |
| PC20, mg/mL | 2.11±1.50† | 4.59±0.09 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download