Abstract
Purpose
Although bronchiolitis obliterans (BO) most often occurs after infection, the incidence of post-transplant BO has recently increased due to the increase of organ and bone marrow transplantation. However, there is limited data on the responses to treatment using measurements of pulmonary function in patients with BO. This study aimed to describe clinical characteristics and pulmonary function in children with BO from a single institute and to compare the responses according to treatment modalities in children with post-infectious BO.
Methods
This study was conducted on 22 children who were diagnosed with BO from January 2005 to December 2010. Based on the medical chart, treatment courses and prognosis of the patients were examined retrospectively. The severity of clinical symptoms was determined by the Denver symptom score, basal pulmonary function, and responses to bronchodilators; all parameters were measured and compared between the time of diagnoses and follow-up six months later.
Results
The mean age of the patients when diagnosed with BO was 8.3 6.6 years; of those ± patients, sixteen were boys and six were girls. Nineteen cases of BO were associated with acute infection, and the most common cause of those cases was adenovirus. Three cases of BO occurred following allogeneic bone marrow transplantation for acute myelogenous leukemia. The Denver symptom scores at the time of diagnosis were averaged to 3.95 0.63, and the average symptom ± score after follow-up of six months was 2.15 0.73. The averages of the % forced vital capacity ± (FVC), % forced expiratory volume in 1 second (FEV1), and % forced expiratory flow, midexpiratory phase (FEF25-75%) at the time of diagnosis were 69 13%, 40.5 12.7%, and 17.6 7.8%, respecti ± ± ± – vely, and FEV1/FVC was 56.7 10.9%. The averages of %FVC, % FEV 1, and %FEF25-75% six months ± after diagnosis were 78 17.3%, 62.5 16.5%, and 35.6 9.5%, respectively, and FEV ± ± ± 1/FVC was improved to 70.7 18.9%. Symptom scores of the group treated with high dose systemic steroids ± decreased significantly compared to those of the group treated with inhaled corticosteroids (P< 0.05). Likewise, improvement of FEV1/FVC after treatment was greater in the group treated with high dose systemic steroids than in the group treated with inhaled corticosteroids (P<0.05).
Go to : 
References
2. Castro-Rodriguez JA, Daszenies C, Garcia M, Meyer R, Gonzales R. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: a 5-year follow-up. Pediatr Pulmonol. 2006; 41:947–53.

3. Kurland G, Michelson P. Bronchiolitis obliterans in children. Pediatr Pulmonol. 2005; 39:193–208.

4. Mattiello R, Mallol J, Fischer GB, Mocelin HT, Rueda B, Sarria EE. Pulmonary function in children and adolescents with postinfectious bronchiolitis obliterans. J Bras Pneumol. 2010; 36:453–9.
5. Fischer GB, Sarria EE, Mattiello R, Mocelin HT, Castro-Rodriguez JA. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev. 2010; 11:233–9.

6. Fan LL, Kozinetz CA. Factors influencing survival in children with chronic interstitial lung disease. Am J Respir Crit Care Med. 1997; 156(3 Pt 1):939–42.

7. Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005; 60:13–6.

8. Yoon KA, Lim HS, Koh YY, Kim H. Normal predicted values of pulmonary function test in Korean school-aged children. J Korean Pediatr Soc. 1993; 36:25–37.
9. Hong SJ, Kim BS, Ahn KM, Lee SI, Kim KE, Lee KY, et al. Multicenter study of bronchiolitis obliterans in Korean children. Pediatr Allergy Respir Dis. 2002; 12:136–45.
10. Zhang L, Irion K, Kozakewich H, Reid L, Camargo JJ, da Silva Porto N, et al. Clinical course of postinfectious bronchiolitis obliterans. Pediatr Pulmonol. 2000; 29:341–50.

11. Kim CK, Kim SW, Kim JS, Koh YY, Cohen AH, Deterding RR, et al. Bronchiolitis obliterans in the 1990s in Korea and the United States. Chest. 2001; 120:1101–6.

12. Paz HL, Crilley P, Patchefsky A, Schiffman RL, Brodsky I. Bronchiolitis obliterans after autologous bone marrow transplantation. Chest. 1992; 101:775–8.

13. Murtagh P, Giubergia V, Viale D, Bauer G, Pena HG. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr Pulmonol. 2009; 44:450–6.

14. Kramer MR, Stoehr C, Whang JL, Berry GJ, Sibley R, Marshall SE, et al. The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: low yield of transbronchial lung biopsy. J Heart Lung Transplant. 1993; 12:675–81.
15. Yoo Y, Suh DI, Kim DK, Yu J, Koh YY, Kim CK. Association of HRCT findings suggestive of bronchiolitis obliterans with bronchial Hyperresponsiveness after Mycoplasma pneumoniae pneumonia. Pediatr Allergy Respir Dis. 2004; 14:350–7.
16. Mattiello R, Sarria EE, Stein R, Fischer GB, Mocelin HT, Barreto SS, et al. Functional capacity assessment in children and adolescents with postinfectious bronchiolitis obliterans. J Pediatr (Rio J). 2008; 84:337–43.

17. Aguerre V, Castaños C, Pena HG, Grenoville M, Murtagh P. Postinfectious bronchiolitis obliterans in children: clinical and pulmonary function findings. Pediatr Pulmonol. 2010; 45:1180–5.

18. Cazzato S, Poletti V, Bernardi F, Loroni L, Bertelli L, Colonna S, et al. Airway inflammation and lung function decline in childhood postinfectious bronchiolitis obliterans. Pediatr Pulmonol. 2008; 43:381–90.

19. Champs NS, Lasmar LM, Camargos PA, Marguet C, Fischer GB, Mocelin HT. Post-infectious bronchiolitis obliterans in children. J Pediatr (Rio J). 2011; 87:187–98.

20. Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991; 58:145–9.

21. Khalid M, Al Saghir A, Saleemi S, Al Dammas S, Zeitouni M, Al Mobeireek A, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005; 25:490–3.

22. Oh JH, Kim KC, Kim SW, Hyun DS, Lee SC, Bae SH, et al. Improvement of pulmonary function after administration of azithromycin in a patient with bronchiolitis obliterans: a case report. Tuberc Respir Dis. 2008; 65:410–5.

23. Ratjen F, Rjabko O, Kremens B. High-dose corticosteroid therapy for bronchiolitis obliterans after bone marrow transplantation in children. Bone Marrow Transplant. 2005; 36:135–8.

24. Fullmer JJ, Fan LL, Dishop MK, Rodgers C, Krance R. Successful treatment of bronchiolitis obliterans in a bone marrow transplant patient with tumor necrosis factor-alpha blockade. Pediatrics. 2005; 116:767–70.
Go to : 
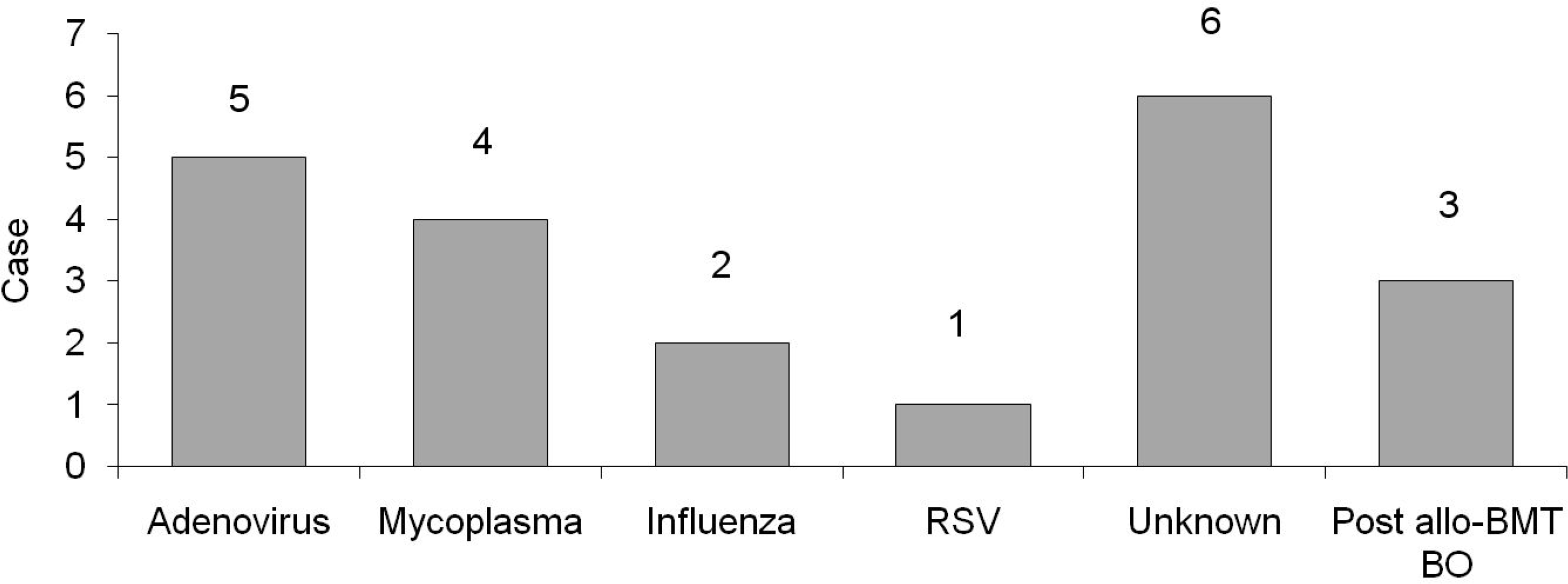 | Fig. 1.Underlying causes in the patients with bronchiolitis obliterans. RSV, respiratory syncytial virus; allo-BMT BO, allogenic bone marrow transplantation bonchiolitis obliterans. |
Table 1.
Clinical Datas of the Patients with Bronchiolitis Obliterans
Table 2.
Pulmonary Function Data in the Patients with Bronchiolitis Obliterans
Table 3.
Comparison of Clinical Symptom and Pulmonary Function between High-Dose Steroid Treatment Group (HST) and non High-Dose Steroid Treatmetn Group (non HST) in the Patients with Post-Infectious Bronchiolitis Obliterans




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download