Abstract
Purpose
There has been a scarcity of integrated, longterm (>4 week) studies on structural and functional alterations in the penis according to the period following cavernous nerve (CN) injury. The aim of this study was to investigate time-dependent structural and functional changes in the corpus cavernosum following CN injury in a rat model.
Materials and Methods
Ninety male Sprague-Dawley rats (10 weeks old) were divided into 4 groups: normal control (C), sham (S), bilateral CN resection (R), and bilateral CN crush injury (I) groups. At 1, 4, and 12 weeks after the procedure, erectile function was assessed by electrostimulation. The terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate nick end labeling (TUNEL) assay was performed for detection of apoptosis. Masson's trichrome staining and immunohistochemistry were performed for detection of alpha smooth muscle actin (α-SMA). Western blot analysis was then performed.
Results
The R and I groups showed persistent impairment of erectile function at all three points in time. Apoptosis peaked at 1 week after resection or crush injury and then gradually subsided. The smooth muscle cell/collagen ratio and expression of α-SMA gradually decreased over time after CN resection or crush injury. Myosin phosphatase target subunit 1 phosphorylation progressively increased over time after CN resection or crush injury. On the other hand, expression of phospho-protein kinase B, phospho-endothelial nitric oxide synthase, and neuronal nitric oxide synthase transiently decreased at 1 week after resection or crush injury and then recovered to the control values.
Go to : 
REFERENCES
1). Montorsi F, Burnett AL. Erectile dysfunction after radical prostatectomy. BJU Int. 2004; 93:1–2.

2). Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000; 55:58–61.

3). Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008; 18:613–20.

4). Mulhall JP, Slovick R, Hotaling J, Aviv N, Valenzuela R, Waters WB, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol. 2002; 167:1371–5.

5). User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003; 169:1175–9.

6). Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009; 6:415–28.

7). Mulhall JP, Graydon RJ. The hemodynamics of erectile dysfunction following nerve-sparing radical retropubic prostatectomy. Int J Impot Res. 1996; 8:91–4.
8). Mulhall JP, Müller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008; 5:1126–36.

9). Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, et al. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009; 56:716–25.

10). Gratzke C, Strong TD, Gebska MA, Champion HC, Stief CG, Burnett AL, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J Urol. 2010; 184:2197–204.

11). Cho MC, Park K, Chai JS, Lee SH, Kim SW, Paick JS. Involvement of sphingosine-1-phosphate/RhoA/Rho-kinase signaling pathway in corporal fibrosis following cavernous nerve injury in male rats. J Sex Med. 2011; 8:712–21.

12). Jin HR, Chung YG, Kim WJ, Zhang LW, Piao S, Tuvshintur B, et al. A mouse model of cavernous nerve injury-induced erectile dysfunction: functional and morphological characterization of the corpus cavernosum. J Sex Med. 2010; 7:3351–64.

13). Park K, Lee DG, Kim SW, Paick JS. Dimethylarginine dimethylaminohydrolase in rat penile tissue: reduced enzyme activity is responsible for erectile dysfunction in a rat model of atherosclerosis. Int J Impot Res. 2009; 21:228–34.

14). Park K, Ryu KS, Li WJ, Kim SW, Paick JS. Chronic treatment with a type 5 phosphodiesterase inhibitor suppresses apoptosis of corporal smooth muscle by potentiating Akt signalling in a rat model of diabetic erectile dysfunction. Eur Urol. 2008; 53:1282–8.

15). Li WJ, Park K, Paick JS, Kim SW. Chronic treatment with an oral rho-kinase inhibitor restores erectile function by suppressing corporal apoptosis in diabetic rats. J Sex Med. 2011; 8:400–10.

16). Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by longterm sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005; 68:226–32.

17). Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006; 3:77–83.

18). Kim HJ, Kim HY, Kim SY, Lee SH, Lee WK, Yang DY. Spontaneous recovery of cavernous nerve crush injury. Korean J Urol. 2011; 52:560–5.

19). Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007; 4:908–16.

20). Zhou H, Zhang KX, Li YJ, Guo BY, Wang M, Wang M. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses high glucose-induced proliferation and collagen synthesis in rat cardiac fibroblasts. Clin Exp Pharmacol Physiol. 2011; 38:387–94.
21). Washida N, Wakino S, Tonozuka Y, Homma K, Tokuyama H, Hara Y, et al. Rho-kinase inhibition ameliorates peritoneal fibrosis and angiogenesis in a rat model of peritoneal sclerosis. Nephrol Dial Transplant. 2011; 26:2770–9.

22). Takeda Y, Nishikimi T, Akimoto K, Matsuoka H, Ishimitsu T. Beneficial effects of a combination of Rho-kinase inhibitor and ACE inhibitor on tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Hypertens Res. 2010; 33:965–73.

23). Satoh S, Yamaguchi T, Hitomi A, Sato N, Shiraiwa K, Ikegaki I, et al. Fasudil attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Eur J Pharmacol. 2002; 455:169–74.

24). Zhou H, Li YJ, Wang M, Zhang LH, Guo BY, Zhao ZS, et al. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2011; 32:999–1008.

25). Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 2004; 17:29–34.

26). Musicki B, Burnett AL. eNOS function and dysfunction in the penis. Exp Biol Med (Maywood). 2006; 231:154–65.

27). Jung GW, Kwak JY, Yoon S, Yoon JH, Lue TF. IGF-I and TGF-beta2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int J Impot Res. 1999; 11:247–59.
Go to : 
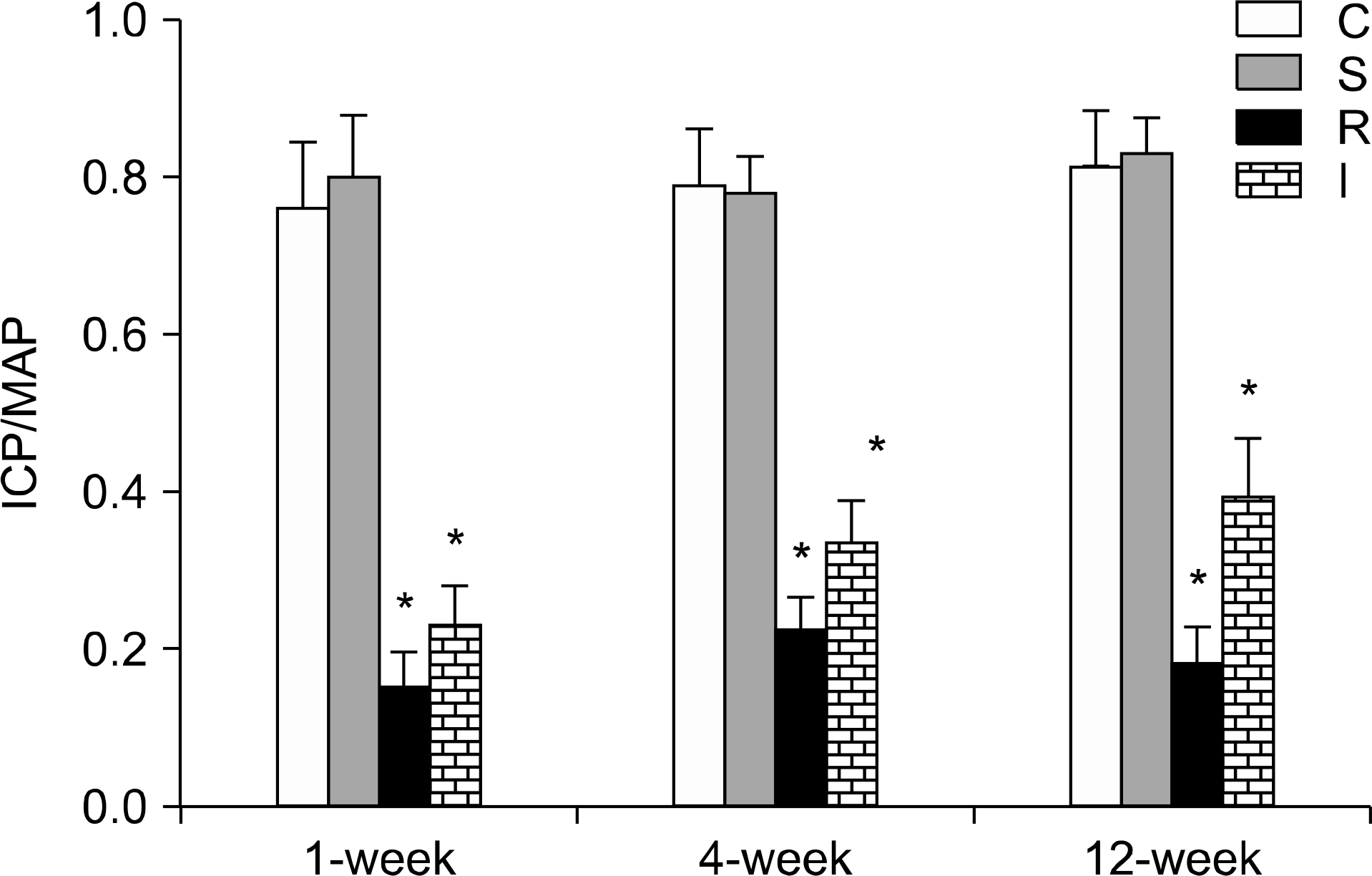 | Fig. 1.Comparison of erectile function. Results of cavernous electrostimulation in the four experimental groups are expressed as percent ICP/MAP. Each bar represents the mean± standard error of the means. ∗p<0.05 vs. control group. ICP: intracavernous pressure, MAP: mean arterial pressure, C: control group (n=5 in each subgroup), S: sham operation group (n=5 in each subgroup), R: CN resection group (n=10 in each of the 1-, 4-, and 12-week subgroups), I: CN crush injury group (n=10 in each of the 1-, 4-, and 12-week subgroups). |
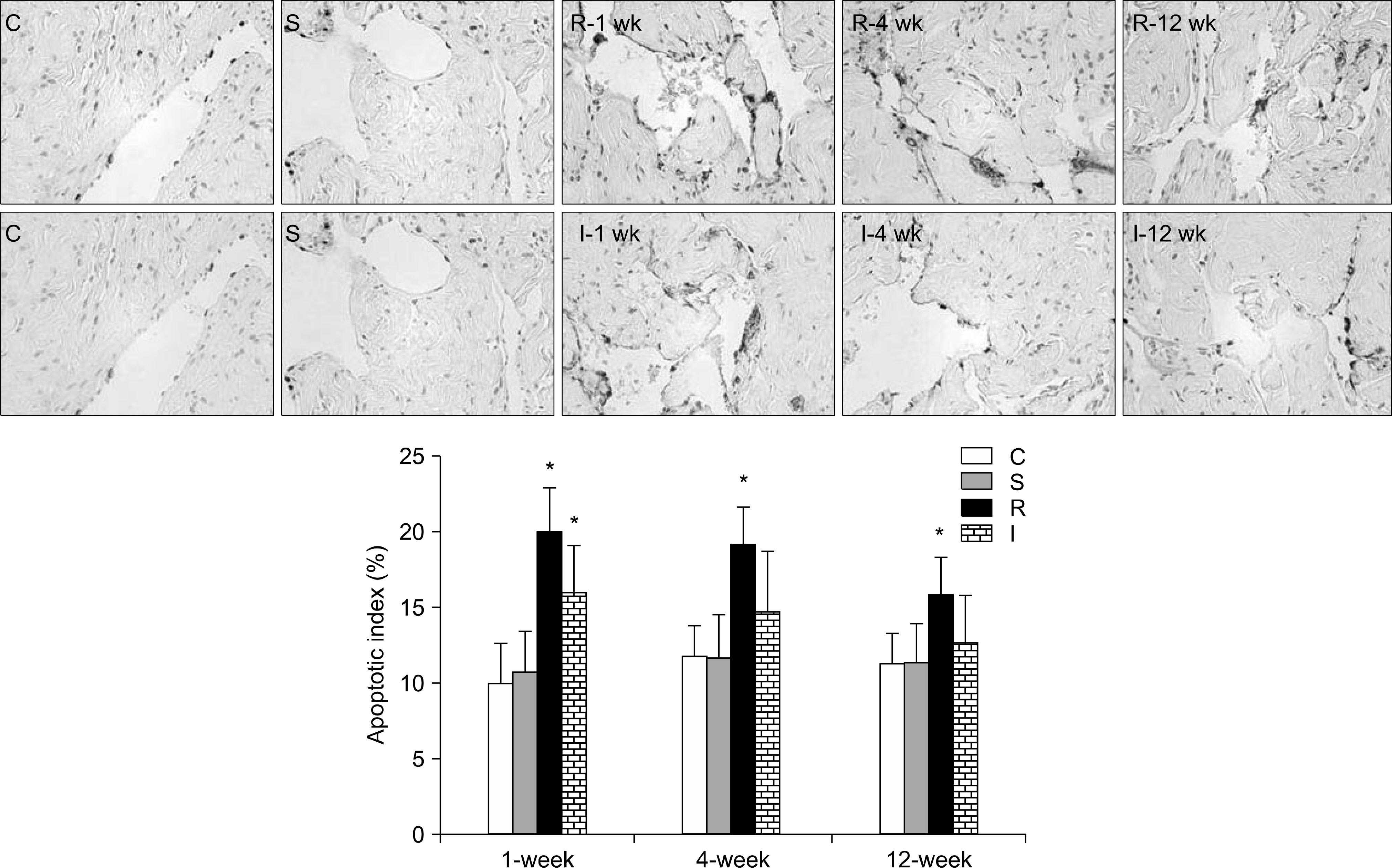 | Fig. 2.Comparison of mean apoptosis index. Representative micrographs show apoptotic cells stained black-brown by the TUNEL method in rat penile cavernosum (magnification 400×). Bar graphs represent quantitative image analysis. The apoptotic index was defined as the percentage of apoptotic cells within the total number of cells in a given area. ∗p<0.05 vs. control group. TUNEL: terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick end labeling, C: control group (n=5 in each subgroup), S: sham operation group (n=5 in each subgroup), R: CN resection group (n=10 in each of the 1-, 4-, and 12-week subgroups), I: CN crush injury group (n=10 in each of the 1-, 4-, and 12-week subgroups). |
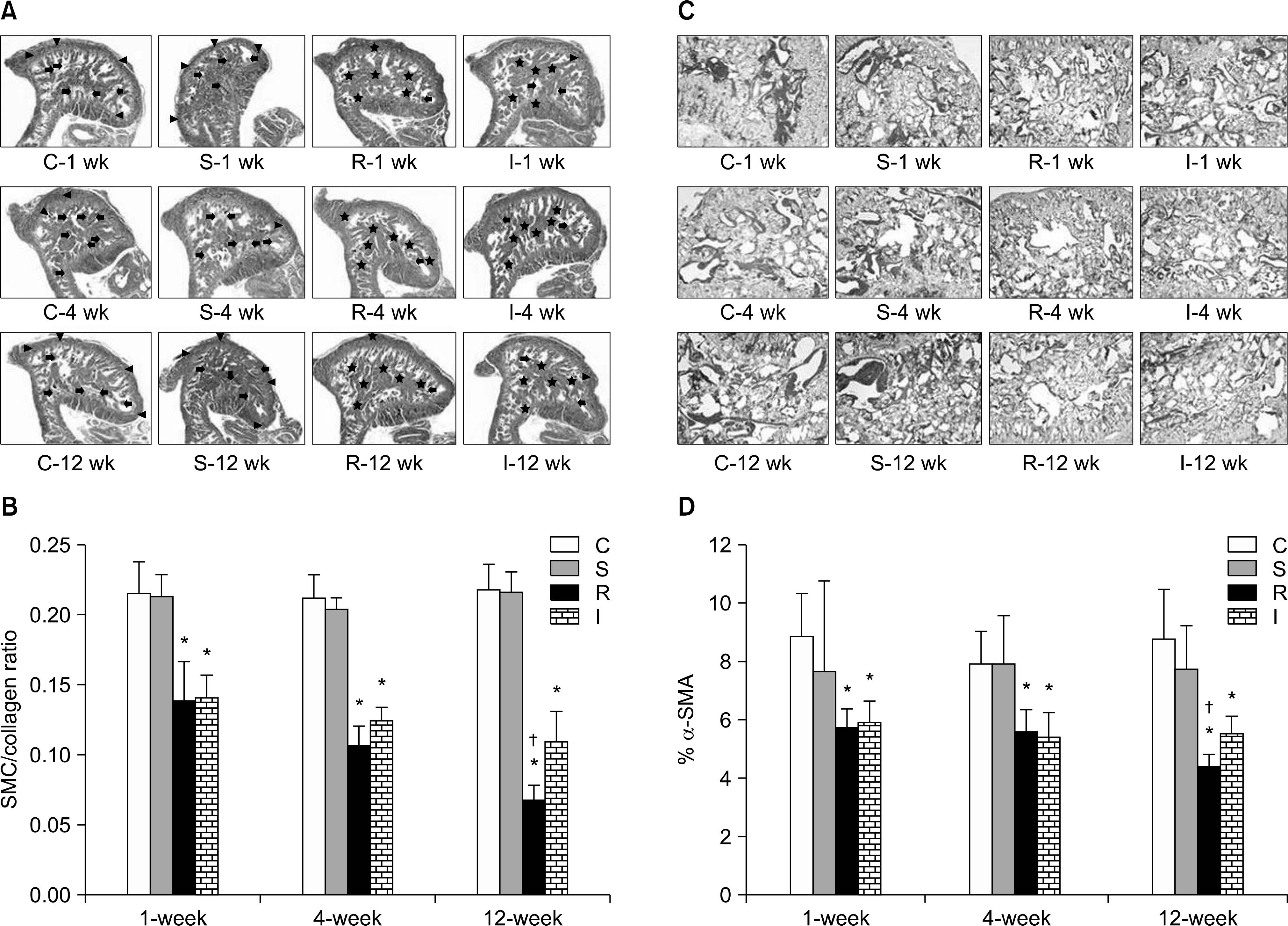 | Fig. 3.Masson's trichrome staining and immunohistochemical analysis of smooth muscle content in rat penile sections. Smooth muscle and collagen fibers were stained in red and blue, respectively (magnification 40×). (A) Representative images for Masson's trichrome staining. (B) Comparison of Masson's trichrome staining results among four experimental groups. The results are presented as the smooth muscle cell (SMC)/collagen ratio (mean±standard error of the means). Smooth muscle cells in the endothelial lining of sinusoids (black arrows), SMCs in the cavernous tissue beneath the tunica albuginea (black-head arrows), collagen fibers in the peri-sinusoidal area or the cavernous tissue beneath the tunica albuginea (asterisks). The smooth muscle component is shown as brown areas (magnification 100×). (C) Representative images for immune-stained alpha smooth muscle actin (α-SMA). (D) Comparison of the expression of α-SMA among four experimental groups. The results are presented as the percentage of smooth muscle fibers in a given area. ∗p<0.05 vs. control group, †p<0.05 vs. crush injury group. C: control group (n=5 in each subgroup), S: sham operation group (n=5 in each subgroup), R: cavernous nerve (CN) resection group (n=10 in each of the 1-, 4-, and 12-week subgroups), I: CN crush injury group (n=10 in each of the 1-, 4-, and 12-week subgroups). |
 | Fig. 4.Western blot analysis demonstrating corporal expression of phospho-myosin phosphatase target subunit 1 (MYPT1), phospho-protein kinase B (Akt), phospho-endothelial nitric oxide synthase (eNOS), and neuronal nitric oxide synthase (nNOS). (A) Representative immunoblots show expression of phospho-MYPT1, phospho-Akt, total Akt, phospho-eNOS, nNOS, and β-actin from the corporal tissues of the four groups (according to period). (B) Bar graphs demonstrating the comparison of phospho-MYPT1 protein expression among the four experimental groups using densitometry. Results were normalized by β-actin expression. (C) Bar graphs demonstrating the comparison of phospho-Akt protein expression among the four experimental groups using densitometry. Results were normalized by total Akt expression. (D) Bar graphs demonstrating the comparison of phospho-eNOS protein expression among the four experimental groups using densitometry. Results were normalized by β-actin expression. (E) Bar graphs demonstrating the comparison of nNOS protein expression among the four experimental groups using densitometry. Results were normalized by β-actin expression. Results were presented as fold changes over controls. ∗p<0.05 vs control group. C: control group (n=5 in each subgroup), S: sham operation group (n=5 in each subgroup), R: cavernous nerve (CN) resection group (n=10 in each of the 1-, 4-, and 12-week subgroups), I: CN crush injury group (n=10 in each of the 1-, 4-, and 12-week subgroups, respectively). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download