Abstract
There is strong evidence from multiple epidemiological studies that benign prostate hyperplasia (BPH) induced lower urinary tract symptoms (LUTS) are correlated with erectile dysfunction (ED). Although a direct causal relationship is not established yet, four pathophysiological mechanisms can explain the relationship. These include alteration in activity of nitric oxide (NO)-cyclic GMP signal pathway, autonomic hyperactivity, increased Rho kinase/Rho A pathway and pelvic atherosclerosis. Androgens have been suggested to have an important role in the maintenance of the functional and structural integrity of the urinary tract. Sexual function should be assessed and discussed with the patient when choosing the appropriate management strategy for LUTS, as well as when evaluating the patient's response to treatment. Multiple large clinical trials have shown an improvement in LUTS after phosphodiesterase-5 (PDE5)-inhibitor treatment. Sildenafil is a pioneer of this clinical trial and appears to improve both erectile function and LUTS in subjects with ED. Basically PDE5 I with long half life is an appropriate candidate, therefore tadalafil and undenafil had been used to evaluate both diseases. Placebo-controlled trials of tadalafil showed improvement of LUTS secondary to BPH, but none of the studies showed a significant effect on urodynamic measures. PDE5 Is, such as sildenafil and tadalafil, increase the concentration of cGMP in plasma and smooth muscle, facilitating erection of the penis, relaxation of the bladder neck and prostate and subsequent bladder emptying. And theses PDE5 Is increase cAMP and cGMP levels and are more highly distributed in the prostate than plasma. These findings may help in the assessment of the feasibility of using PDE5 Is to concurrently treat both LUTS and ED.
Go to : 
REFERENCES
1). Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991; 338:469–71.

2). Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61.

3). Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–9.

4). Laborde EE, McVary KT. Medical management of lower urinary tract symptoms. Rev Urol. 2009; 11(Suppl 1):S19–25.
5). Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res. 2000; 12:305–11.

6). Girman CJ, Jacobsen SJ, Rhodes T, Guess HA, Roberts RO, Lieber MM. Association of health-related quality of life and benign prostatic enlargement. Eur Urol. 1999; 35:277–84.

7). Burnett AL, Maguire MP, Chamness SL, Ricker DD, Takeda M, Lepor H, et al. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995; 45:435–9.

8). Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol. 2008; 26:603–9.

9). Baltaci S, Orhan D, Gögüs C, Türkölmez K, Tulunay O, Gögüs O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001; 88:100–3.

10). Uckert S, Küthe A, Jonas U, Stief CG. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001; 166:2484–90.
11). Khan MA, Thompson CS, Dashwood MR, Mumtaz FH, Morgan RJ, Mikhailidis DP. Endothelin-1 and nitric oxide in the pathogenesis of urinary tract disorders secondary to bladder outlet obstruction. Curr Vasc Pharmacol. 2003; 1:27–31.

12). Andersson KE, Chapple CR, Höfner K. Future drugs for the treatment of benign prostatic hyperplasia. World J Urol. 2002; 19:436–42.

13). Kaplan SA, Gonzalez RR. Phosphodiesterase type 5 inhibitors for the treatment of male lower urinary tract symptoms. Rev Urol. 2007; 9:73–7.
14). Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008; 180:1228–34.

15). Roumeguère T, Zouaoui Boudjeltia K, Hauzeur C, Schulman C, Vanhaeverbeek M, Wespes E. Is there a rationale for the chronic use of phosphodiesterase-5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia? BJU Int. 2009; 104:511–7.

16). Köhler TS, McVary KT. The relationship between erectile dysfunction and lower urinary tract symptoms and the role of phosphodiesterase type 5 inhibitors. Eur Urol. 2009; 55:38–48.

17). Hotston M, Shukla N, Bloor J, Persad R, Jeremy JY. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006; 98:1331–2.

18). Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol. 2008; 26:603–9.

19). Richter K, Heuer O, Uckert S, Stief CG, Jonas U, Wolf G. Immunocytochemical distribution of nitric oxide synthases in the human prostate. J Urol Suppl. 2004; 171:: abstract 347.
20). Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997; 33:1–8.

21). Lin G, Huang YC, Wang G, Lue TF, Lin CS. Prominent expression of phosphodiesterase 5 in striated muscle of the rat urethra and levator ani. J Urol. 2010; 184:769–74.

22). McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, McKenna KE. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 1994; 51:99–107.
23). Björntorp P, Rosmond R. The metabolic syndrome–a neuroendocrine disorder? Br J Nutr. 2000; 83(Suppl 1):S49–57.
24). Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998; 83:1853–9.

25). Uckert S, Sormes M, Kedia G, Scheller F, Knapp WH, Jonas U, et al. Effects of phosphodiesterase inhibitors on tension induced by norepinephrine and accumulation of cyclic nucleotides in isolated human prostatic tissue. Urology. 2008; 71:526–30.

26). Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001; 7:119–22.

27). Chitaley K, Bivalacqua TJ, Champion HC, Usta MF, Hellstrom WJ, Mills TM, et al. Adeno-associated viral gene transfer of dominant negative RhoA enhances erectile function in rats. Biochem Biophys Res Commun. 2002; 298:427–32.

28). Rees RW, Foxwell NA, Ralph DJ, Kell PD, Moncada S, Cellek S. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003; 170:2517–22.

29). Chang S, Hypolite JA, Zderic SA, Wein AJ, Chacko S, DiSanto ME. Enhanced force generation by corpus cavernosum smooth muscle in rabbits with partial bladder outlet obstruction. J Urol. 2002; 167:2636–44.

30). Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, et al. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol. 2003; 285:F990–7.

31). Hale TM, Okabe H, Bushfield TL, Heaton JP, Adams MA. Recovery of erectile function after brief aggressive antihypertensive therapy. J Urol. 2002; 168:348–54.

32). Tarcan T, Azadzoi KM, Siroky MB, Goldstein I, Krane RJ. Age-related erectile and voiding dysfunction: the role of arterial insufficiency. Br J Urol. 1998; 82(Suppl 1):26–33.

33). Ponholzer A, Temml C, Wehrberger C, Marszalek M, Madersbacher S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol. 2006; 50:581–6.

34). Kim SO, Son KC, Im CM, Jung SI, Kwon DD, Park KS, et al. The effects of risk factors for vascular disease on LUTS and erectile dysfunction. Eur Urol Suppl. 2008; 7:131.

35). Azadzoi KM, Tarcan T, Siroky MB, Krane RJ. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999; 161:1626–35.

36). Kozlowski R, Kershen RT, Siroky MB, Krane RJ, Azadzoi KM. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001; 165:1019–26.

37). Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008; 26:359–64.

38). Rosenzweig BA, Bolina PS, Birch L, Moran C, Marcovici I, Prins GS. Location and concentration of estrogen, progesterone, and androgen receptors in the bladder and urethra of the rabbit. Neurourol Urodyn. 1995; 14:87–96.

39). Salmi S, Santti R, Gustafsson JA, Mäkelä S. Co-localization of androgen receptor with estrogen receptor beta in the lower urinary tract of the male rat. J Urol. 2001; 166:674–7.
40). Keast JR. The autonomic nerve supply of male sex organs–an important target of circulating androgens. Behav Brain Res. 1999; 105:81–92.
41). Chamness SL, Ricker DD, Crone JK, Dembeck CL, Maguire MP, Burnett AL, et al. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995; 63:1101–7.
42). Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology. 2007; 69:708–13.

43). Ponholzer A, Madersbacher S. Lower urinary tract symptoms and erectile dysfunction; links for diagnosis, management and treatment. Int J Impot Res. 2007; 19:544–50.

44). Tinel H, Stelte-Ludwig B, Hütter J, Sandner P. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006; 98:1259–63.

45). Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010; 7:524–40.

46). Sairam K, Kulinskaya E, McNicholas TA, Boustead GB, Hanbury DC. Sildenafil influences lower urinary tract symptoms. BJU Int. 2002; 90:836–9.

47). Chang HS, Park CH, Kim CI, Kim KS, Kim DG, Seo YJ, et al. Influence of sildenafil on lower urinary tract symptoms. Eur Urol. 2006; 5(Suppl):178.

48). Mulhall JP, Guhring P, Parker M, Hopps C. Assessment of the impact of sildenafil citrate on lower urinary tract symptoms in men with erectile dysfunction. J Sex Med. 2006; 3:662–7.

49). McVary KT, Monnig W, Camps JL Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007; 177:1071–7.

50). McVary KT, Siegel RL, Carlsson M. Sildenafil citrate improves erectile function and lower urinary tract symptoms independent of baseline body mass index or LUTS severity. Urology. 2008; 72:575–9.

51). Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2007; 51:1717–23.

52). McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007; 177:1401–7.

53). Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008; 180:1228–34.

54). Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol. 2010; 183:1092–7.

55). Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008; 53:1236–44.

56). Zhao C, Kim SH, Lee SW, Jeon JH, Kang KK, Choi SB, et al. Activity of phosphodiesterase type 5 inhibitors in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int. 2011; 107:1943–7.

Go to : 
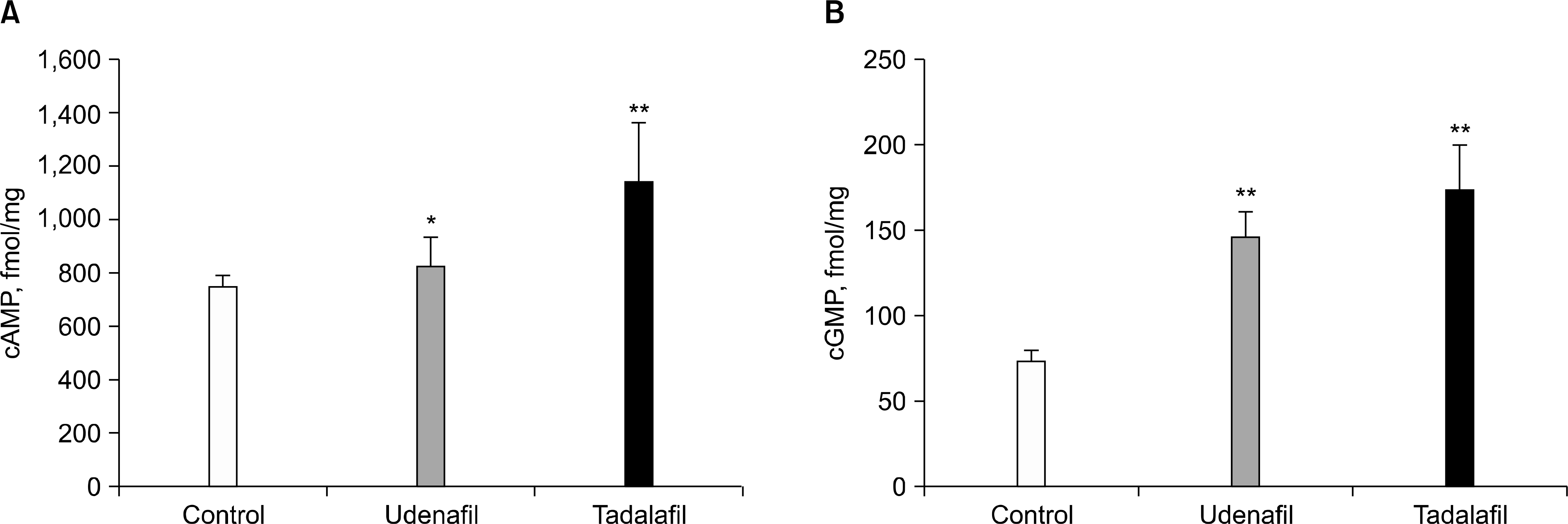 | Fig. 1.cAMP and cGMP levels in prostate tissue. (A) cAMP and (B) cGMP levels were significantly higher in prostate tissues of groups 2 (udenafil, 200 mg) and 3 (tadalafil, 20 mg) than group 1 (control). ∗p<0.05, ∗∗p<0.01. |
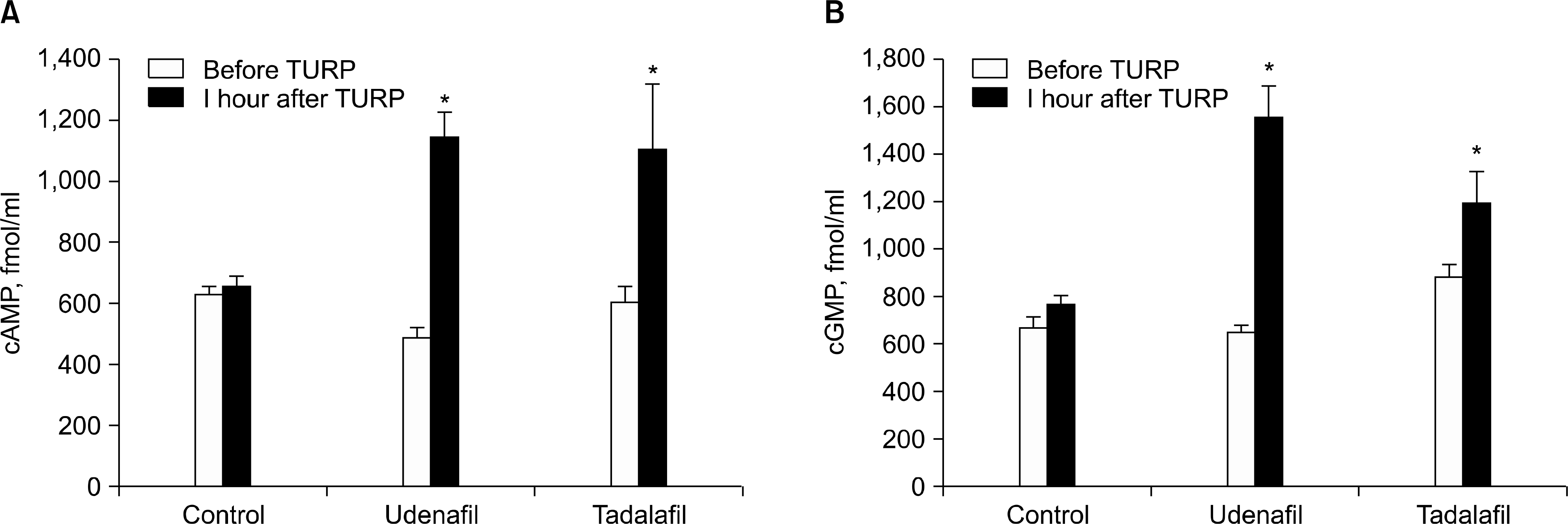 | Fig. 2.cAMP and cGMP levels in plasma. (A) cAMP and (B) cGMP levels increased significantly 1 h after TURP in the plasma of groups 2 (udenafil, 200 mg) and 3 (tadalafil, 20 mg), but not group 1 (control). ∗p<0.01. |
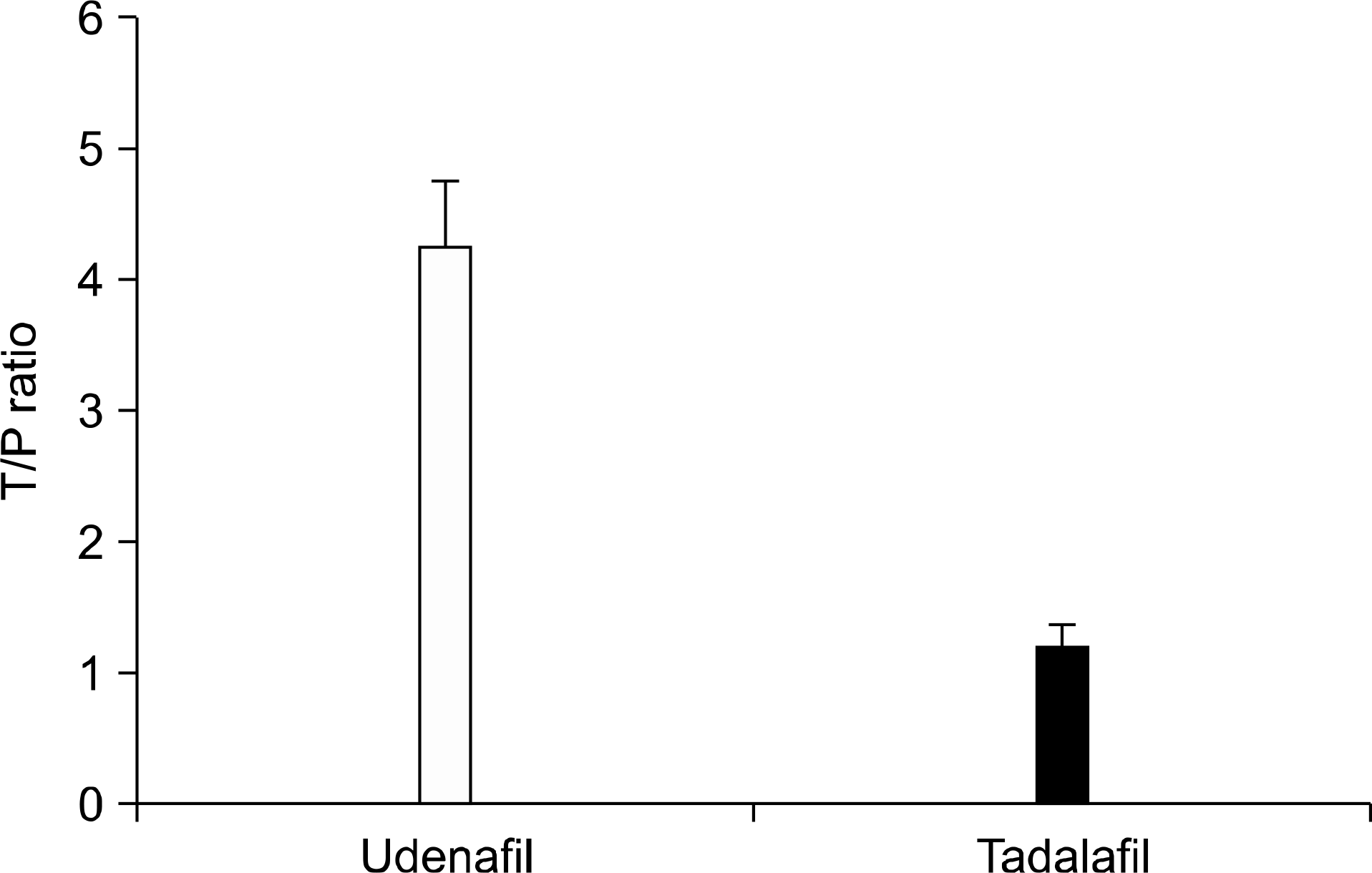 | Fig. 3.The prostate tissue-to-plasma (T/P) ratio of the phosphodiesterase type 5 inhibitor (PDE5 I) concentration. The ratio was significantly higher in group 2 (udenafil, 200 mg) than in group 3 (tadalafil, 20 mg). |
Table 1.
Clinical evidence of sildenafil and lower urinary tract symptoms
Table 2.
Clinical evidence of tadalafil and lower urinary tract symptoms derived from clinical trials




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download