Abstract
Purpose
Varicocele is known as a main cause of primary male infertility and it supposed to be associated with oxidative stress. Anthocyanin is known as a natural plant pigment and novel antioxidant. This study was designed to investigate the effects of anthocyanin on a rat model of varicocele.
Materials and Methods
Twenty four male rats, induced varicocele by partial obstruction of left renal vein, were divided into four experimental groups: the group induced varicocele for four weeks without anthocyanin, the group received anthocyanin (80 mg/kg) right after varicocele induction, group induced varicocele for eight weeks without anthocyanin, and the group received anthocyanin (80 mg/kg) after four weeks observation following varicocele induction. After anthocyanin treatment, testes from the rats in all groups were removed, weighed, and subjected to histological examination. Apoptosis in the testes was measured by the TUNEL assay. And the oxidative stress was evaluated by measurement of 8-hydroxy-2'-deoxyguanosine (8-OHdG).
Results
Induction of varicocele led to decreasing left testis weight, decreasing spermatogenic cell density significantly (p<0.05). Also it led to increasing apoptotic body counts and increasing concentration of 8-OHdG significantly (p<0.05). However administration of anthocyanin right after varicocele induction prevent this change meaningfully (p<0.05). In group received anthocyanin after four weeks observation following varicocele induction, interestingly, there was no significant difference in testis weight, spermatogenic cell density, apoptotic body count and concentration of 8-OHdG compared to group induced varicocele for eight weeks without anthocyanin administration.
Conclusions
These results suggest that anthocyanin is effective in decreasing the oxidative stress of testis in rat induced varicocele and may be effective in making a healthy sperm in patient of varicocele in early stage. However in patient under way in advanced stage, it is supposed that the anthocyanin cannot help having a protective effect from oxidative stress narrowly unless the condition of oxidative stress by varicocele is corrected. Further studies are needed to better understand the mechanisms and actions of anthocyanin and varicocele, and these studies may lead to the clinical application of anthocyanin in preventing male infertility by varicocele.
REFERENCES
1). Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993; 59:613–6.
2). Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001; 7:473–81.
3). Hendin BN, Kolettis PN, Sharma RK, Thomas AJ Jr, Agarwal A. Varicocele is associated with elevated spermatozoa reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999; 161:1831–4.
4). Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol. 1999; 17:296–300.

5). Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, et al. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat mode. J Agric Food Chem. 2010; 58:12686–91.
6). Saypol DC, Howards SS, Turner TT, Miller ED Jr. Influence of surgically induced varicocele in testicular blood flow, temperature, and histology in adult rats and dogs. J Clin Invest. 1981; 68:39–45.
7). Goldstein M. Surgical management of male infertility and other scrotal disorders. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell's urology. 7th ed.Philadelphia: Saunders;1998. p. 1384–71.
8). Hagood PG, Mehan DJ, Worischeck JH, Andrus GH, Parra RO. Laparoscopic varicocelectomy: preliminary report of a new technique. J Urol. 1992; 147:73–6.

9). Ralph DJ, Timoney AG, Parker C, Pryor JP. Laparoscopic varicocele ligation. Br J Urol. 1993; 72:230–3.

10). Jarow JP, Assimos DG, Pittaway DE. Effectiveness of laparoscopic varicocelectomy. Urology. 1993; 42:544–7.

11). Turek PJ, Lipshultz LI. The varicocele controversies II: diagnosis and management. AUA Update Series. 1995; 14:114–9.
12). Cohen MS, Plaine L, Brown IS. The role of internal spermatic vein plasma catecholamine determination in sub-fertile men with varicoceles. Fertil Steril. 1975; 26:1243–9.
13). Goldstein M, Eid JF. Elevation of intratesticular and scrotal temperature in men with varicocele. J Urol. 1989; 142:743–5.
14). Shin JW, Kim SW, Paick JS. Effect of varicocele treatments in adolescents: changes of semen parameters after early varicocelectomy. Korean J Urol. 2005; 46:481–6.
15). Lyon RP, Marshall S, Scott MP. Varicocele in childhood and adolescence implication in adulthood infertility? Urology. 1982; 19:641–4.

16). Park SP, Nam HJ, Park HJ, Park NC. Correlation between duration of varicocele and testicular damage in an experimental rat model. Korean J Androl. 2009; 27:18–24.
17). Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996; 48:835–50.

18). Aitken RJ, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW. Differential contribution of leukocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates oligozoospermic patients and fertile donors. J Reprod Fertil. 1992; 94:451–62.
19). de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997; 2:48–54.

20). Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987; 81:459–69.

21). Atessahin A, Karahan I, Türk G, Gür S, YIlmaz S, ÇeribasI AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol. 2006; 21:42–7.
22). Ateşşahin A, Türk G, Karahan I, Yilmaz S, Ceribaşi AO, Bulmuş O. Lycopene prevents adriamycin-induced testicular toxicity in rats. Fertil Steril. 2006; 85:1216–22.
23). Türk G, Ateşşahin A, Sönmez M, Yüce A, Ceribaşi AO. Lycopene protects against cyclosporine A-induced testicular toxicity in rats. Theriogenology. 2007; 67:778–85.

24). Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, et al. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart diseases. Am J Cardiol. 2005; 96:810–4.
25). Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006; 187:363–71.

26). Sönmez M, Türk G, Yüce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology. 2005; 63:2063–72.

27). Sönmez M, Yüce A, Türk G. The protective effects of melatonin and vitamin E on antioxidant enzyme activities and epididymal sperm characteristics of homocysteine treated male rats. Reprod Toxicol. 2007; 23:226–31.

28). Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem. 2000; 48:5677–84.

29). Shih PH, Yeh CT, Yen GC. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem Toxicol. 2005; 55:1557–66.

30). de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997; 2:48–54.

31). Henkel R. The impact of oxidants on sperm functions. Andrologia. 2005; 37:205–6.
32). Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005; 84:850–3.

Fig. 1.
(A) It shows ligation of proximal renal vein with plastic probe. Black arrow indicates plastic probe and white arrow indicates the engorgement of left gonadal vein and distal renal vein. (B) It shows partial obstruction of proximal renal vein after removal of plastic probe. Gray arrow indicates engorged left gonadal vein.

Fig. 2.
Histopathologic findings of left testis (H&E stain). The thickness of germinal cell layer (B) is more increased than that (A) statistically (×400). (C) and (D) necrosis of germinal cells was observed and the thickness of germinal cell layer was more decreased than that (B). And there was no difference in spermatogenic cell density between (C) and (D) statistically (×400). (A) Group induced varicocele for 4 weeks without anthocyanin administration. (B) Group received oral dose of anthocyanin (80 mg/kg) for 4 weeks right after varicocele induction. (C) Group induced varicocele for 8 weeks without anthocyanin administration. (D) Group received oral dose of anthocyanin (80 mg/kg) for 4 weeks after 4 weeks observation following varicocele induction.

Fig. 3.
Mean weight of testes in each groups. Left testis weight of group received anthocyanin right after varicocele induction is significantly higher than group induced varicoele for 4 weeks without anthocyanin administration statistically (p<0.05). But there was no difference between group induced varicocele for 8 weeks without anthocyanin and group received anthocyanin after 4 weeks observation following varicocele induction statistically (p>0.05). ∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) right after varicocele induction. ∗∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) after 4 weeks observation following varicocele induction.
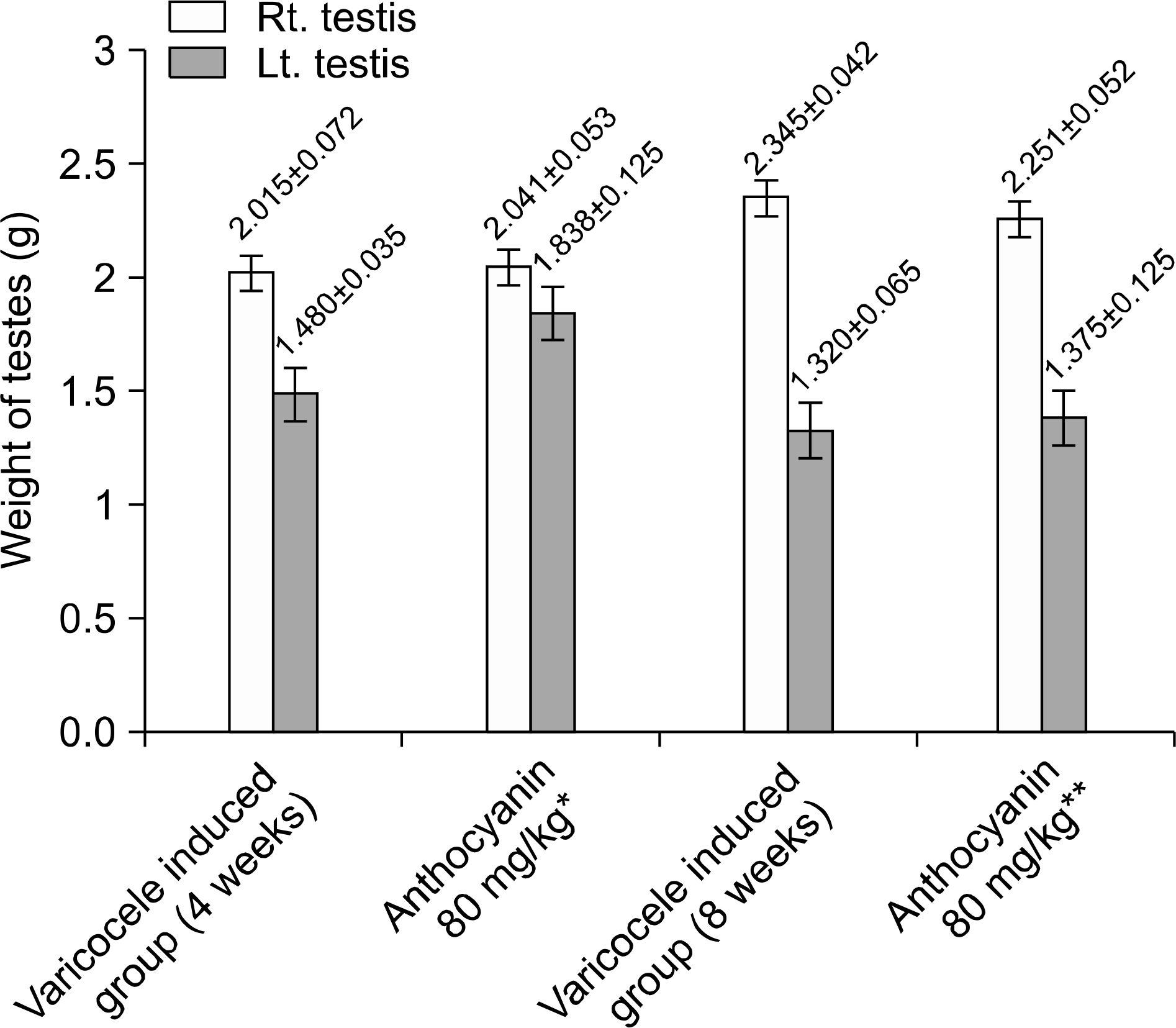
Fig. 4.
Spermatogenic cell density (germinal cell layer thickness/ diameter of seminiferous tubule). Spermatogenic cell density of group received anthocyanin right after varicocele induction is significantly higher than group induced varicocele varicocele for 4 weeks without anthocyanin administration statistically (p <0.05). But there was no difference between group induced varicocele for 8 weeks without anthocyanin administration and group received anthocyanin after 4 weeks observation following varicocele induction statistically (p>0.05). ∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) right after varicocele induction. ∗∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) after 4 weeks observation following varicocele induction.
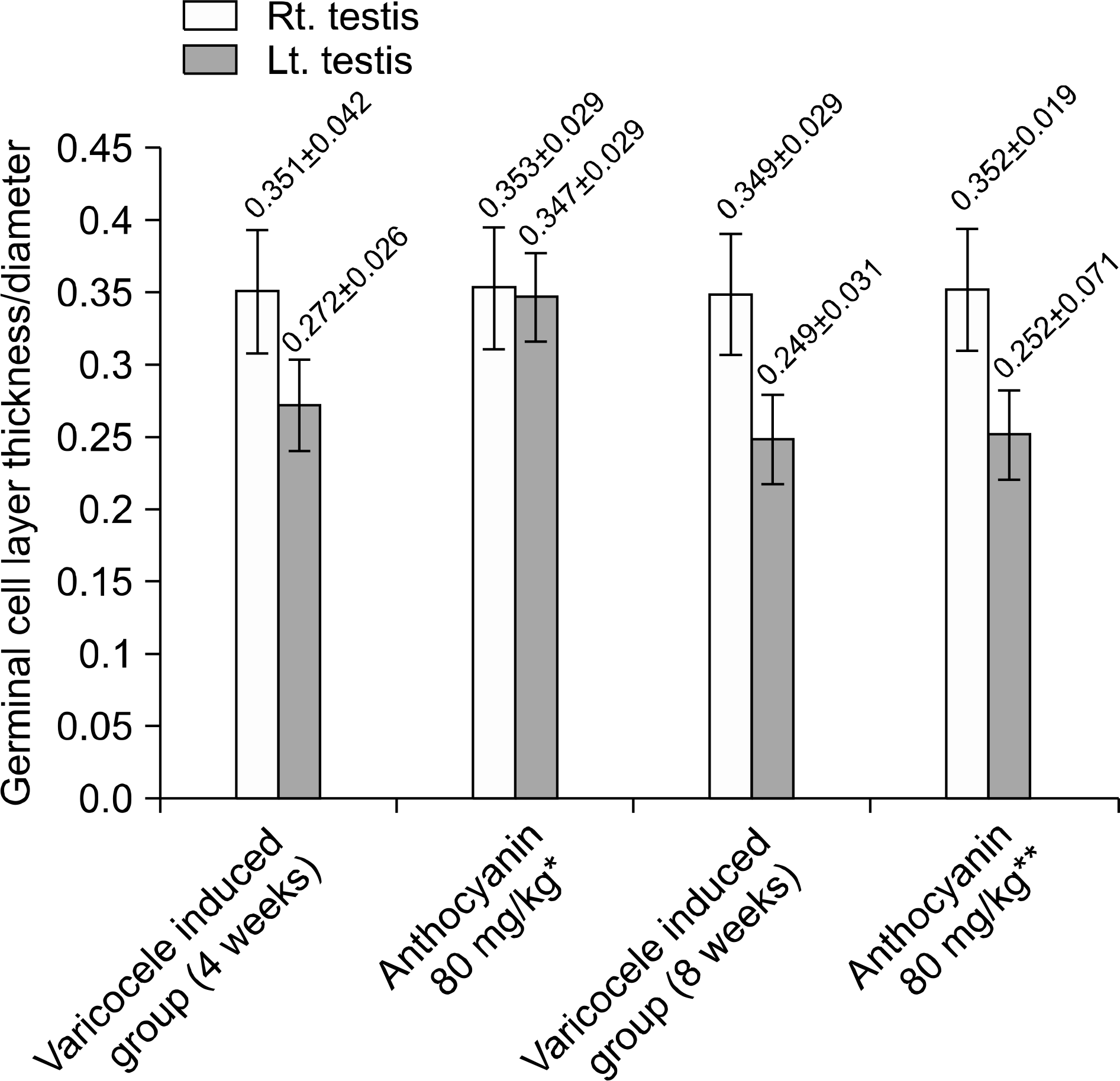
Fig. 5.
Apoptotic bodies in TUNEL stain of left testis. Positive TUNEL stain cell called apoptotic body was showed in dark brown or black color in TUNEL stain (×200). (A) Counts of apoptotic bodies was more increased than that (B). (C) and (D) apoptotic bodies were more increased than that (A). But there was no difference in counts of apoptotic bodies between (C) and (D) statistically (×200). (A) Group induced varicocele for 4 weeks without anthocyanin administration. (B) Group received oral dose of anthocyanin (80 mg/kg) for 4 weeks right after varicocele induction. (C) Group induced varicocele for 8 weeks without anthocyanin administration. (D) Group received oral dose of anthocyanin (80 mg/kg) for 4 weeks after 4 weeks observation following varicocele induction.

Fig. 6.
Numbers of apoptotic bodies. In group received anthocyanin right after varicocele induction, count of apoptotic bodies is significantly lower than that of group induced varicocele for 4 weeks without anthocyanin administration statistically (p<0.05). But there was no difference between group induced varicocele for 8 weeks without anthocyanin. administration and group received anthocyanin after 4 weeks observation following varicocele induction statistically (p> 0.05). ∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) right after varicocele induction. ∗∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) after 4 weeks observation following varicocele induction
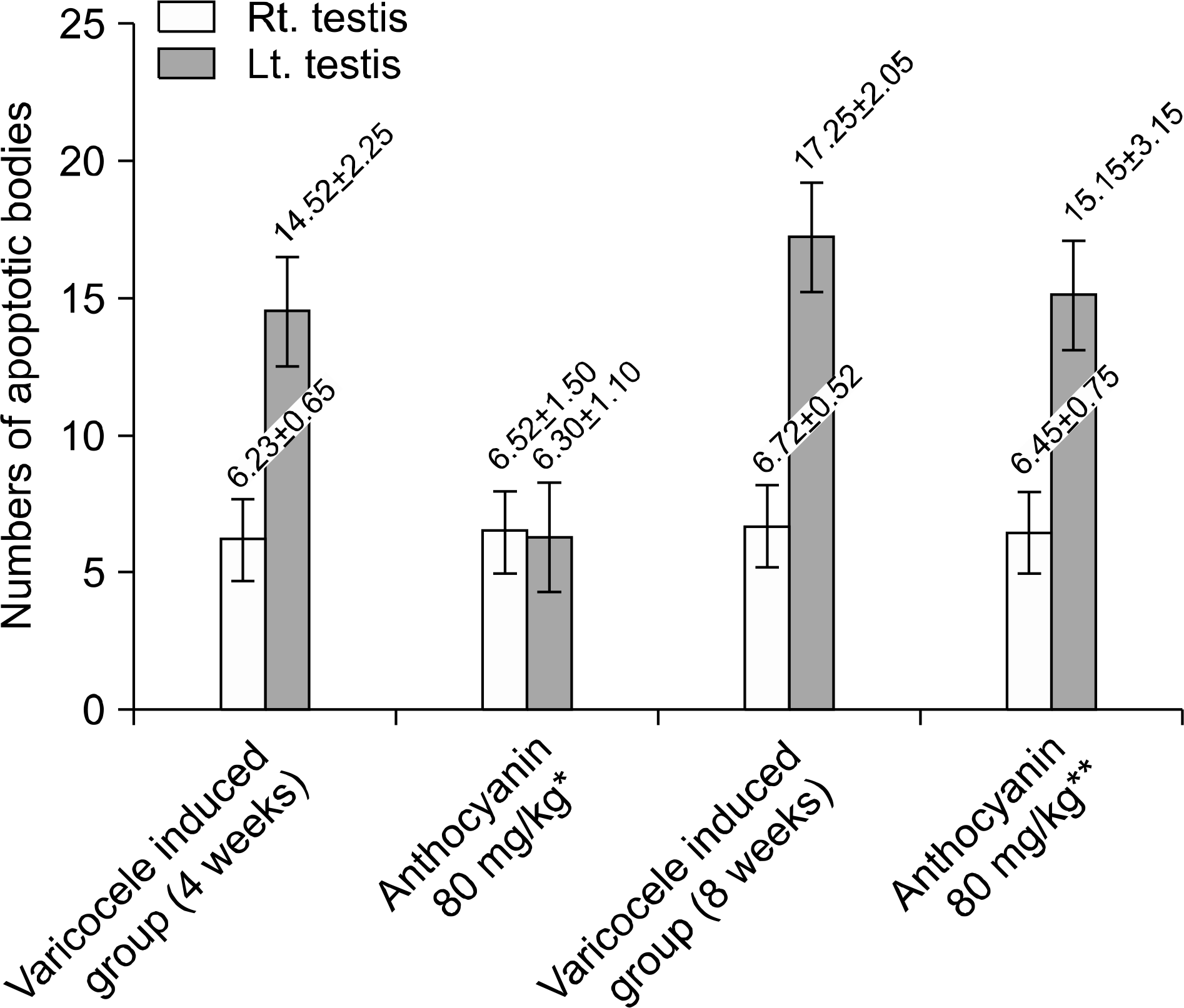
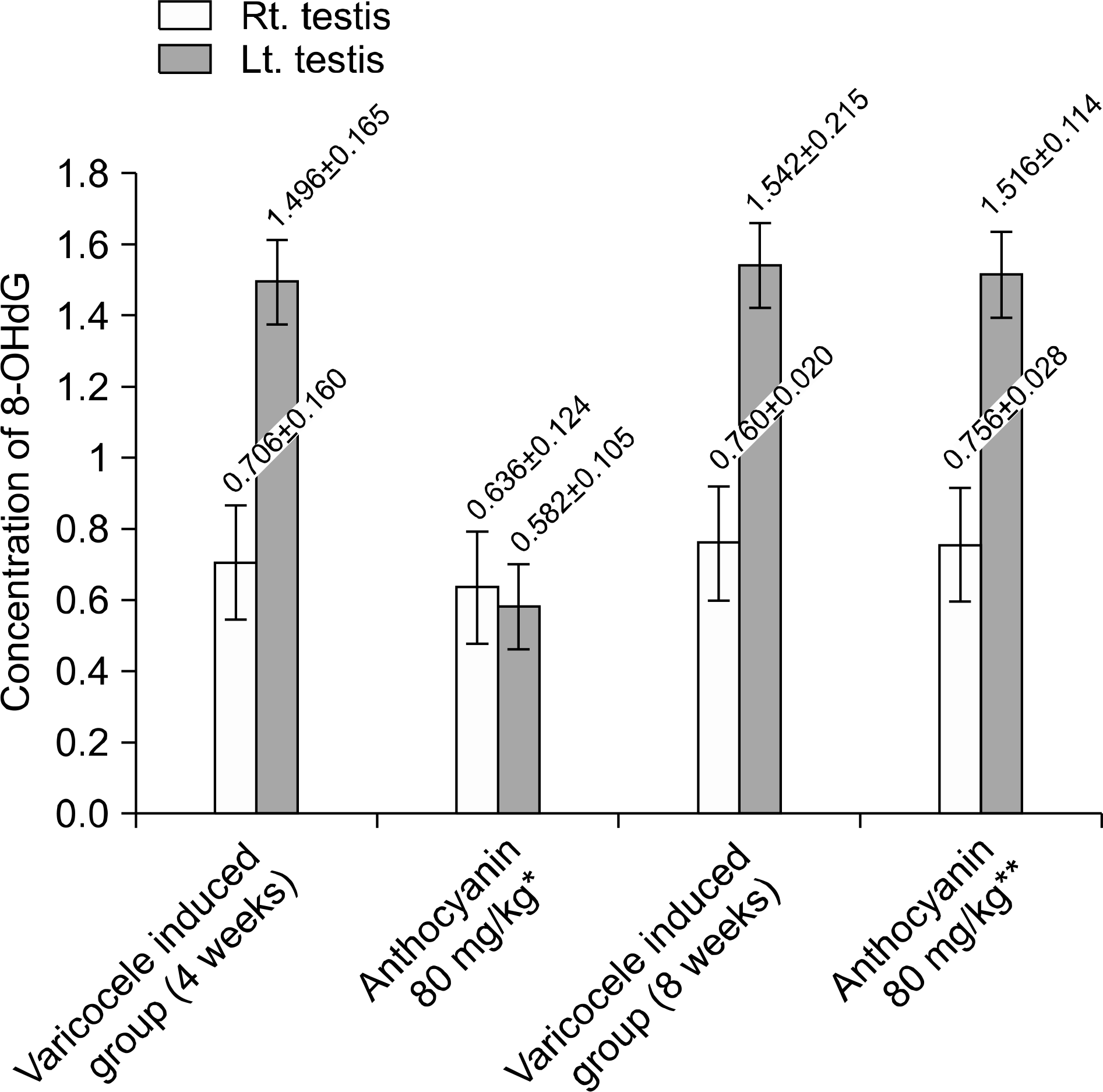
Fig. 7.
Concentration of 8-OHdG (8-hydroxy-2'-deoxyguanosine). In group received anthocyanin right after varicocele induction, concentration of 8-OHdG is significantly lower than that of group induced varicocele for 4 weeks without anthocyanin administration statistically (p<0.05). But there was no difference between group induced varicocele for 8 weeks without anthocyanin administration and group received anthocyanin after 4 weeks observation following varicocele induction statistically (p>0.05). ∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) right after varicocele induction. ∗∗Anthocyanin 80 mg/kg: group received oral dose of anthocyanin (80 mg/kg) after 4 weeks observation following varicocele induction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download