Abstract
Purpose
We compared the efficacy and safety profiles of dose increase, traditional combination methods, and combining different alpha blockers in hypertensive males with lower urinary tract symptom (LUTS) refractory to an initial dose of 4 mg doxazosin.
Materials and Methods
Between 2000 and 2005, 374 male patients with LUTS and hypertension unresponsive to 4 weeks of 4 mg doxazosin were enrolled. The subjects were randomly classified into 3 groups, 8 mg/day of doxazosin (D group), 4 mg of doxazosin plus 0.2 mg/day of tamsulosin (DT group), and 4 mg doxazosin plus 5 mg/day finasteride (DF group). Patients were evaluated based on their International Prostate Symptom Score (IPSS), quality of life (QOL), uroflowmetry and blood pressure (BP) and adverse events (AEs) at the baseline and 3 and 12 months after treatment.
Results
The 269 patients (71.9%) were followed for at least 1 year (D group n=84, DT group n=115, and DF group n=70). The clinical parameters before and after initial 4 mg/day doxazosin were not different among the 3 groups. IPSS improvement after 3 months and maximal flow rate (Qmax) improvement after 3 and 12 months were significantly higher in the D and DT groups than the DF group (p<0.05). Sitting systolic and diastolic BP of the D group decreased larger than those of the other 2 groups (p<0.05). At least one of the AEs was reported by 29.0%, 19.3%, and 17.3% of patients in the D, DT, and DF groups, respectively. In particular, vasodilatory AEs of the D group (28.2%) were higher than those of other groups (p<0.05), and sexual function AEs of the DF group (10.9%) were higher than those of other groups (p<0.05).
Go to : 
REFERENCES
1). Holtgrewe HL, Mebust WK, Dowd JB, Cockett AT, Peters PC, Proctor C. Transurethral prostatectomy: practice aspects of the dominant operation in American urology. J Urol. 1989; 141:248–53.

2). Souverein PC, Erkens JA, de la Rosette JJ, Leufkens HG, Herings RM. Drug treatment of benign prostatic hyperplasia and hospital admission for BPH-related surgery. Eur Urol. 2003; 43:528–34.

3). McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The longterm effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–98.

4). AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003; 170:530–47.
5). Kirby RS. Doxazosin in benign prostatic hyperplasia: effects on blood pressure and urinary flow in normotensive and hypertensive men. Urology. 1995; 46:182–6.

6). Chapple CR, Wyndaele JJ, Nordling J, Boeminghaus F, Ypma AF, Abrams P. Tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist. A meta-analysis of two randomized, placebo-controlled, multicentre studies in patients with benign prostatic obstruction (symptomatic BPH). European Tamsulosin Study Group. Eur Urol. 1996; 29:155–67.
7). Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology. 1998; 51:892–900.
8). Boyle P, Napalkov P. The epidemiology of benign prostatic hyperplasia and observations on concomitant hypertension. Scand J Urol Nephrol Suppl. 1995; 168:7–12.
9). Lukacs B, Blondin P, MacCarthy C, Du Boys B, Grippon P, Lassale C. Safety profile of 3 months' therapy with alfuzosin in 13,389 patients suffering from benign prostatic hypertrophy. Eur Urol. 1996; 29:29–35.
10). Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int. 2005; 95(Suppl 4):29–36.
11). Masumori N. Naftopidil for the treatment of urinary symptoms in patients with benign prostatic hyperplasia. Ther Clin Risk Manag. 2011; 7:227–38.

12). Schilit S, Benzeroual KE. Silodosin: a selective al-pha1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia. Clin Ther. 2009; 31:2489–502.
13). Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–9.

14). Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004; 64:1081–8.

15). Roehrborn CG, Prajsner A, Kirby R, Andersen M, Quinn S, Mallen S. A double-blind placebo-controlled study evaluating the onset of action of doxazosin gastrointestinal therapeutic system in the treatment of benign prostatic hyperplasia. Eur Urol. 2005; 48:445–52.

16). Kirby RS. A randomized, double-blind crossover study of tamsulosin and controlled-release doxazosin in patients with benign prostatic hyperplasia. BJU Int. 2003; 91:41–4.

17). Sugaya K, Nishijima S, Miyazato M, Ashitomi K, Hatano T, Ogawa Y. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci Lett. 2002; 328:74–6.

19). Tammela T. Benign prostatic hyperplasia. Practical treatment guidelines. Drugs Aging. 1997; 10:349–66.
20). Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Tsunoo M. Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans. Drug Metab Dispos. 1998; 26:240–5.
21). de Mey C. Cardiovascular effects of alpha-blockers used for the treatment of symptomatic BPH: impact on safety and wellbeing. Eur Urol. 1998; 34(Suppl 2):18–28.

22). Buzelin JM, Fonteyne E, Kontturi M, Witjes WP, Khan A. Comparison of tamsulosin with alfuzosin in the treatment of patients with lower urinary tract symptoms suggestive of bladder outlet obstruction (symptomatic benign prostatic hyperplasia). The European Tamsulosin Study Group. Br J Urol. 1997; 80:597–605.
23). Yamada S, Suzuki M, Tanaka C, Mori R, Kimura R, Inagaki O, et al. Comparative study on alpha 1-adrenoceptor antagonist binding in human prostate and aorta. Clin Exp Pharmacol Physiol. 1994; 21:405–11.
24). De Mey C. Alpha1-blocker therapy for lower urinary tract symptoms suggestive of benign prostatic obstruction: what are the relevant differences in randomised controlled trials? Eur Urol. 2000; 38(Suppl 1):25–39.
25). MacDiarmid SA, Emery RT, Ferguson SF, McGuirt-Franklin R, McIntyre WJ, Johnson DE. A randomized double-blind study assessing 4 versus 8 mg. doxazosin for benign prostatic hyperplasia. J Urol. 1999; 162:1629–32.

26). Abrams P, Speakman M, Stott M, Arkell D, Pocock R. A dose-ranging study of the efficacy and safety of tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist, in patients with benign prostatic obstruction (symptomatic benign prostatic hyperplasia). Br J Urol. 1997; 80:587–96.
27). Okada H, Kamidono S, Yoshioka T, Okuyama A, Ozono S, Hirao Y, et al. A comparative study of terazosin and tamsulosin for symptomatic benign prostatic hyperplasia in Japanese patients. BJU Int. 2000; 85:676–81.

28). Lee E, Lee C. Clinical comparison of selective and non-selective alpha 1A-adrenoreceptor antagonists in benign prostatic hyperplasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol. 1997; 80:606–11.
Go to : 
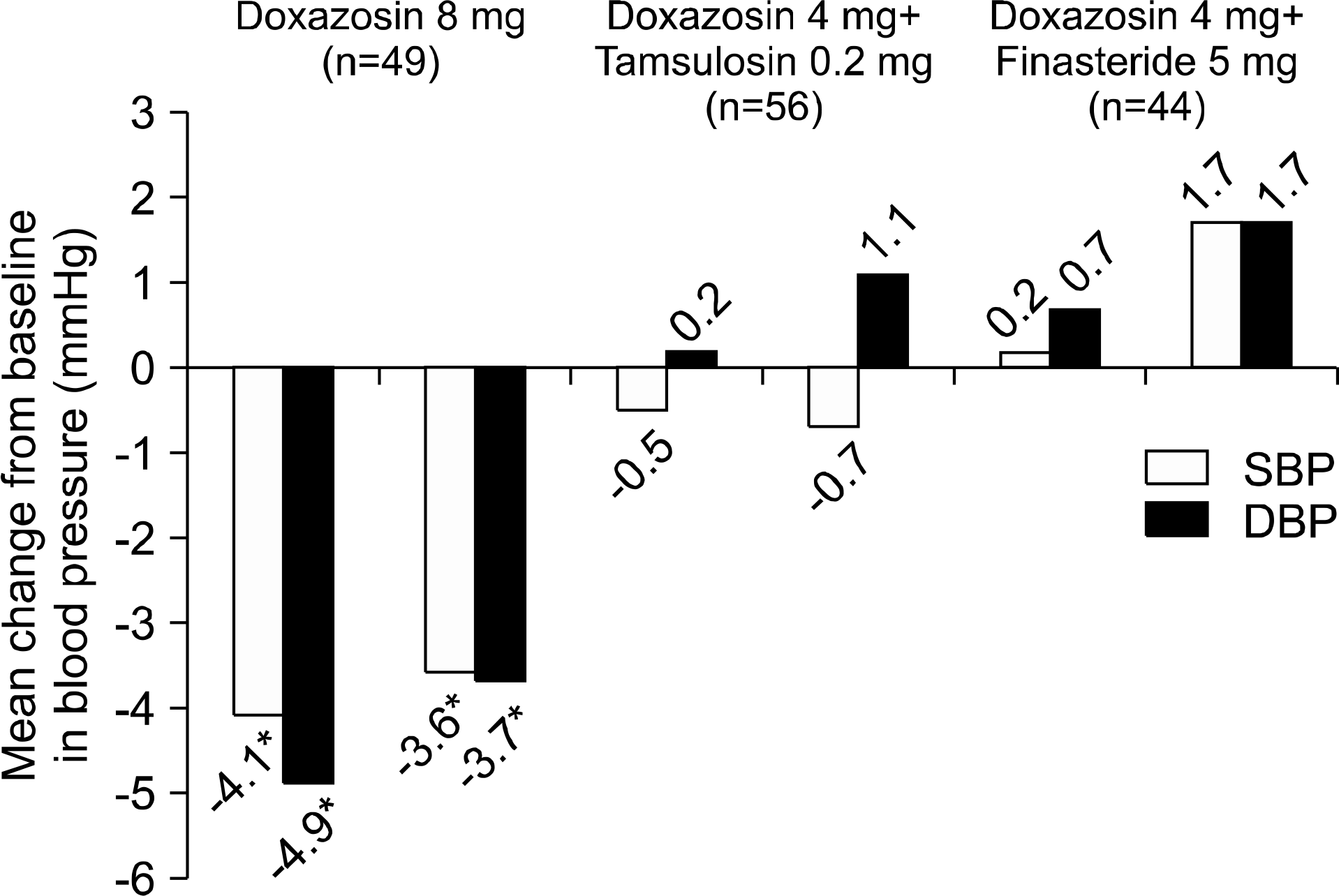 | Fig. 1.Changes in systolic and diastolic blood pressure after 3 and 12 months of dose increase or combination therapy in the 3 groups. The 8 mg doxazosin group after 3 and 12 months of treatment shows a significantly larger BP change than the other groups. SBP: systolic blood pressure, DBP: diastolic blood pressure. ∗Means p<0.05 compared with the other groups. Independent T-test. |
Table 1.
Baseline demographics and characteristics show no difference among groups (mean±SD)
Table 2.
Compared with before-treatment, each group showed improvement of all parameters at 3 and 12 months after the dose increase or beginning of combination therapy (mean±SD)
Table 3.
Compared among groups, the DF group shows less improvement in 3-month IPSS and Qmax and 12-month Qmax parameters (mean±SD)
Table 4.
The incidence of adverse events in all 374 patients enrolled, expressed as number (percentage)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download