Abstract
Purpose
To evaluate the effect and improvement of potassium aminobenzoate (500 mg Peyron capsule) in oral therapy for Peyronie's disease.
Materials and Methods
From February 2011 to September 2011, 31 patients with Peyronie's disease received potassium aminobezoate (500 mg Peyron capsules) and were divided into two groups. Group 1 (N=10) received potassium aminobezoate (500 mg Peyron capsule) 3 g four times daily without previous treatment of Peyronie's disease, while group 2 (N=21) received the same drug with previous treatment of Peyronie's disease (10 mg Tamoxifen +300 mg L-carnitine two times daily). Outcomes were assessed by subjective symptom change, pain relief, resolutions of the plaque, and curvature.
Results
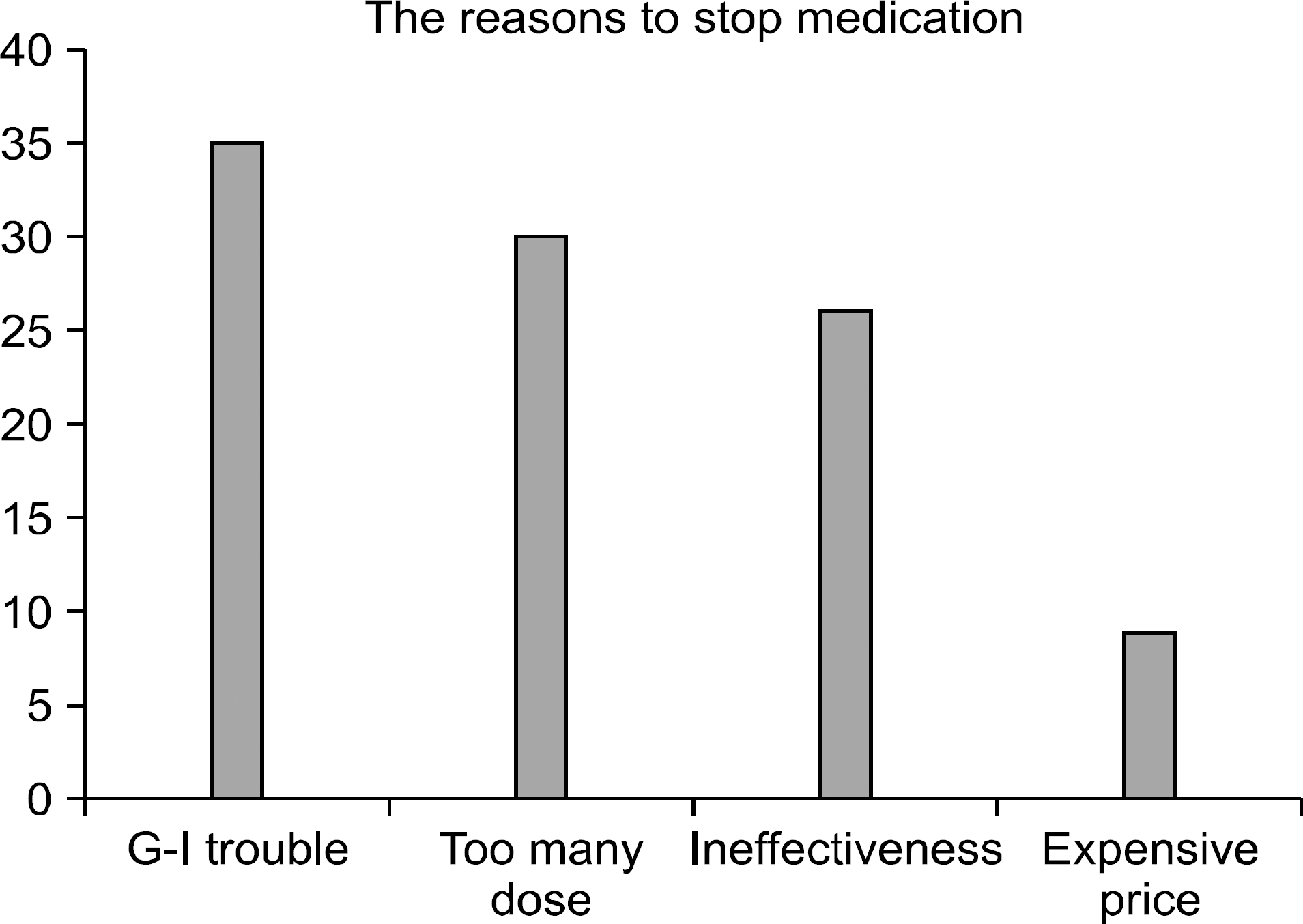
After 3 months, there were no significant improvements in clinical outcomes of either group and among all the patients, 23 stopped taking potassium aminobezoate (23/31, 74%). The reasons for ceasing the therapy were gastrointestinal trouble (8/23, 35%), too many doses to take (7/23, 30%), ineffectiveness (6/23, 26%), and high price (2/23, 9%).
Conclusions
Athough the etiology of Peyronie's disease has not been elucidated, potassium aminobenzoate in therapy of Peyronie's disease has been used. The use of this medication has the limitations of gastrointestinal trouble, ineffectiveness, too many doses, and high price. Further evaluations of the effect and appropriate dosing of potassium aminobenzoate are needed.
Go to : 
REFERENCES
1). Larsen SM, Levine LA. Peyronie's disease: review of nonsurgical treatment options. Urol Clin North Am. 2011; 38:195–205.

2). Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie's disease: results of a large survey. BJU Int. 2001; 88:727–30.

3). Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey'. Int J Impot Res. 2000; 12:305–11.

5). Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997; 158:1388–90.

6). Larsen SM, Levine LA. Review of non-surgical treatment options for Peyronie's Disease. Int J Impot Res 2011.
7). Williams G, Green NA. The non-surgical treatment of Peyronie's disease. Br J Urol. 1980; 52:392–5.

8). Kadioglu A, Tefekli A, Köksal T, Usta M, Erol H. Treatment of Peyronie's disease with oral colchicine: longterm results and predictive parameters of successful outcome. Int J Impot Res. 2000; 12:169–75.

9). Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol. 1999; 162:2003–5.

10). Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int. 2001; 88:63–7.

11). Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium paraaminobenzoate (potaba). J Urol. 1959; 81:770–2.

12). Kierkegaard E, Nielsen B. Peyronie's disease treated with K-paraaminobenzoate and vitamin E. Ugeskr Laeger. 1979; 141:2052–3.
13). Weidner W, Hauck EW, Schnitker J. Peyronie's Disease Study Group of Andrological Group of German Urologists. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol. 2005; 47:530–5.

14). Abern MR, Levine LA. Peyronie's disease: evaluation and review of nonsurgical therapy. Scientific World Journal. 2009; 9:665–75.

15). Jack GS, Gonzalez-Cadavid N, Rajfer J. Conservative management options for Peyronie's disease. Curr Urol Rep. 2005; 6:454–60.

16). Carson CC. Potassium paraaminobenzoate for the treatment of Peyronie's disease: is it effective? Tech Urol. 1997; 3:135–9.
Go to : 
Table 1.
Clinical characteristics of patients before treatment
Table 2.
Clinical characteristics of patients after the treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download