Abstract
Purpose
Adverse sexual experiences such as erectile dysfunction (ED), loss of libido, and ejaculation disorders have been consistent side effects of 5-alpha reductase inhibitors (5ARI). The aim of this study was to evaluate the effects of 5ARI (finasteride) treatment on semen parameters and contraction of the corpus cavernosum and seminal vesicles in male rabbits.
Materials and Methods
Adult male New Zealand White rabbits (n=10) were randomized into 2 groups: finasteride-treatment (5ARI) group and vehicle-treatment (control) group. The 5ARI group received daily oral finasteride (10 mg/day) by gavage for 4∼6 weeks, and the control group received the same concentration of the vehicle. The semen volume and semen parameters between the 2 groups were compared; thereafter, contraction or relaxation responses of smooth muscle strips of the corpus cavernosum and seminal vesicles were observed in an organ bath.
Results
Semen magnesium (14.2 vs 5.1 mg/dl) and protein (2.2 vs 1.6 g/dl) concentrations were significantly lower in the 5ARI group than in the control group. The concentrations of other parameters such as electrolytes (Na/K/Cl), fructose, and citrate did not differ between the 2 groups. The contractile responses to norepinephrine (NE) significantly increased in the 5ARI group compared to the control group and the relaxation responses to acetylcholine (ACh) or sodium nitroprusside (SNP) also increased in the 5ARI group. The contractile responses of the seminal vesicular strips to NE significantly decreased in the 5ARI group compared with the control group.
Go to : 
REFERENCES
1). Gormley GJ. Evaluation of men on finasteride. Semin Urol Oncol. 1996; 14:139–44.
2). Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomizes clinical trials. Urology. 1996; 48:398–405.
3). McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. New Engl J Med. 1998; 338:557–63.
4). Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. New Engl J Med. 1996; 335:553–9.

5). Marberger MJ. Longterm effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. Urology. 1998; 51:677–86.

6). Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007; 4:1708–12.
7). Gormley GJ, Stoner E, Rittmaster RS, Gregg H, Thompson DL, Lasseter KC, et al. Effects of finasteride (MK-906), a 5 alpha-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990; 70:1136–41.
8). Cukierski MA, Sina JL, Prahalada S, Wise LD, Antonello JM, MacDonald JS, et al. Decreased fertility in male rats administered the 5 alpha-reductase inhibitor, finasteride, is due to deficits in copulatory plug formation. Reprod Toxicol. 1991; 5:353–62.
9). Iguer-Ouada M, Verstegen JP. Effect of finasteride (Proscar MSD) on seminal composition, prostate function and fertility in male dogs. J Reprod Fertil Suppl. 1997; 51:139–49.
10). Armbruster DA. Prostate-specific antigen: Biochemistry, analytical methods, and clinical application. Clin Chem. 1993; 39:181–95.

11). Malm J, Hellman J, Magnusson H, Laurell CB, Lilja H. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur J Biochem. 1996; 238:48–53.
12). Veltri R, Rodriguez R. Molecular Biology, Endocrinology, and Physiology of the Prostate and Seminal Vesicles. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed.Philadelphia: Saunders;2007. p. 2677–726.
13). Umeyama T, Ishikawa H, Takeshima H, Yoshii S, Koiso K. A comparative study of seminal trace elements in fertile and infertile men. Fertil Steril. 1986; 46:494–9.

14). Aumüller G, Riva A. Morphology and functions of the human seminal vesicle. Andrologia. 1992; 24:183–96.

15). Driessen B, Bultmann R, Goncalves J, Starke K. Opposite modulation of noradrenaline and ATP release in guinea-pig vas deferens through prejunctional beta-adrenoceptors: evidence for the beta 2 subtype. Naunyn Schmiedebergs Arch Pharmacol. 1996; 353:564–71.

16). Gladkova AI. The regulation of male sexual behavior by the sex hormones. Usp Fiziol Nauk. 1999; 30:97–105.
17). Hong JH. Endocrinologic erectile dysfunction. In: Korean society for sexual medicine and andrology. Textbook of andrology. 2nd ed.Seoul: Koonja;2010. p. 239–46.
18). Li Z, Wang YX, Zheng S, Xiang ZQ, Han YF. The effects of testosterone undecanoate on relaxation of rat corpus cavernosum smooth muscle in vitro. Zhonghua Nan Ke Xue. 2002; 8:130–3.
19). Lugg JA, Rajfer J, Gonzalez-Cadavid NF. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology. 1995; 136:1495–501.

20). Park KH, Kim SW, Kim KD, Paick JS. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999; 83:327–33.

21). Roehrborn CG, Lee M, Meehan A, Waldstreicher J. PLESS Study Group. Effects of finasteride on serum testosterone and body mass index in men with benign prostatic hyperplasia. Urology. 2003; 62:894–9.

22). Waldkirch E, Uckert S, Schultheiss D, Geismar U, Bruns C, Scheller F, et al. Non-genomic effects of androgens on isolated human vascular and nonvascular penile erectile tissue. BJU Int. 2008; 101:71–5.

23). Corradi LS, Goes RM, Carvalho HF, Taboga SR. Inhibition of 5-alpha-reductase activity induces stromal remodeling and smooth muscle de-differentiation in adult gerbil ventral prostate. Differentiation. 2004; 72:198–208.
Go to : 
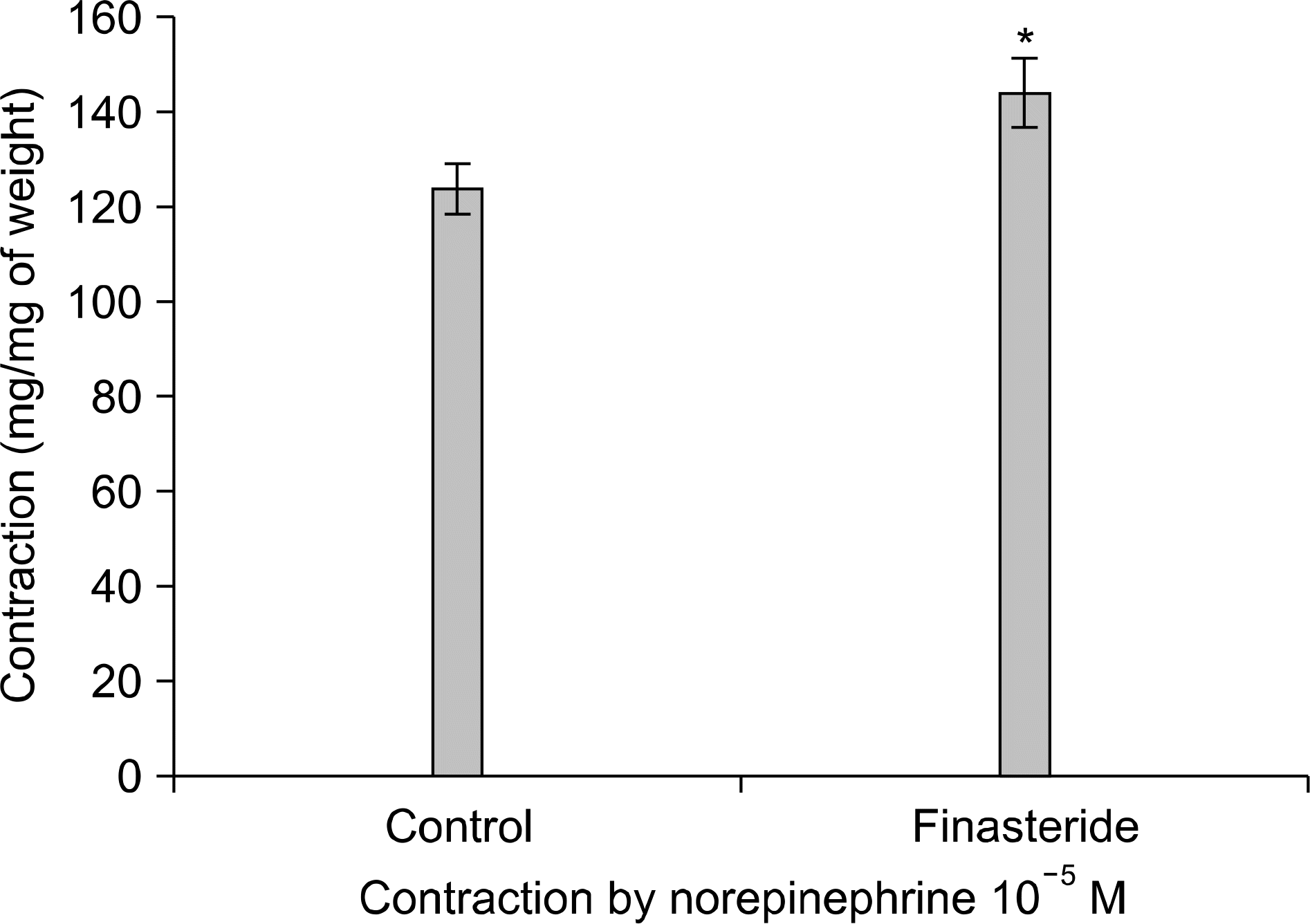 | Fig. 1.Comparison of contraction by norepinephrine 10−5 M on rabbit corpus cavernosal strip according to finasteride medication (n=12, Asterisk indicates p-value is <0.05). Error bars indicate mean±standard error. |
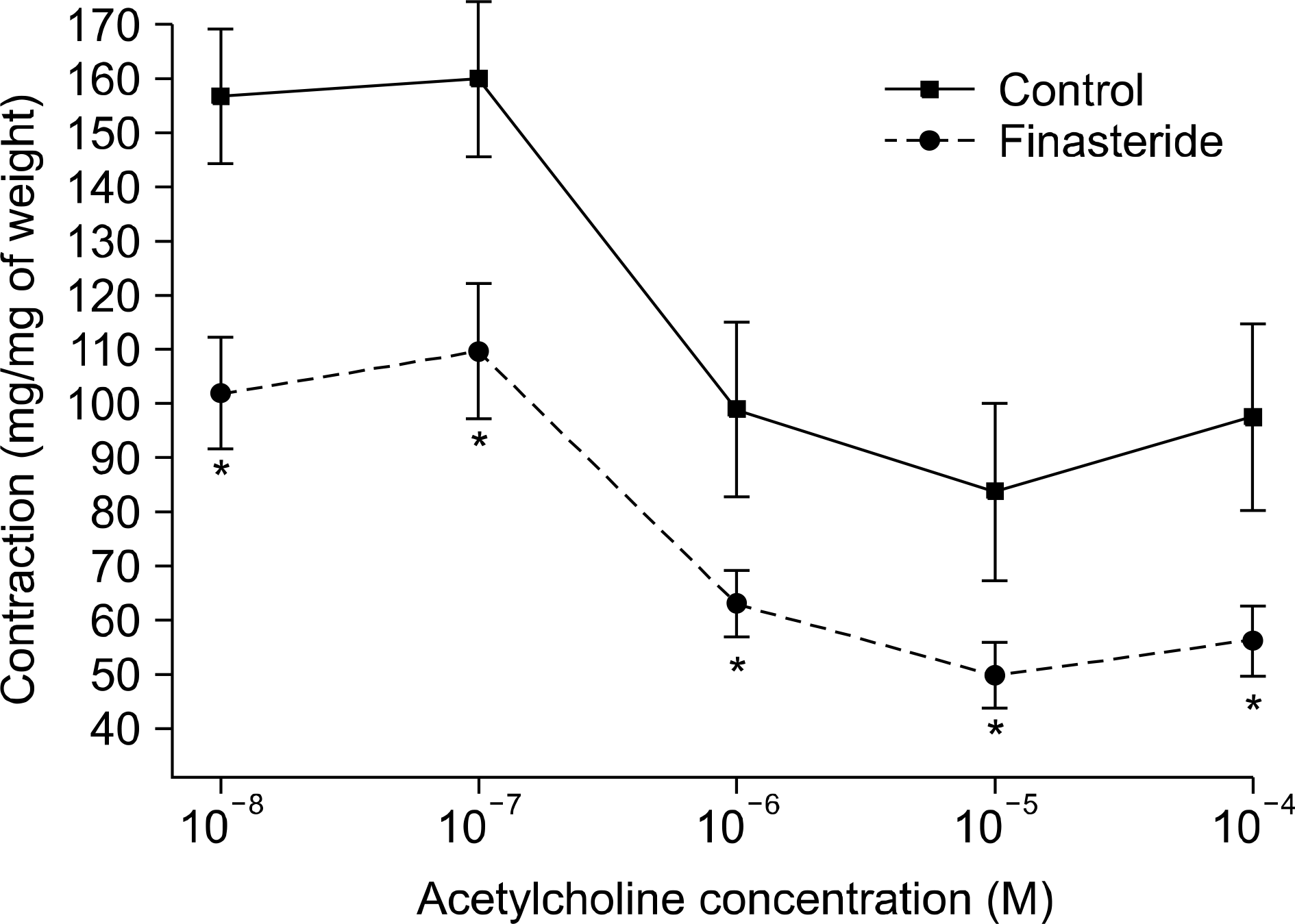 | Fig. 2.Comparison for the effect of finasteride medication on rabbit corpus carvernosal strips relaxation in an acetylcholine dose-dependent manner. The preparation were pre-constricted with 10−5 M norepinephrine (n=12, Asterisk indicates p-values are <0.05). Error bars indicate mean±standard error. |
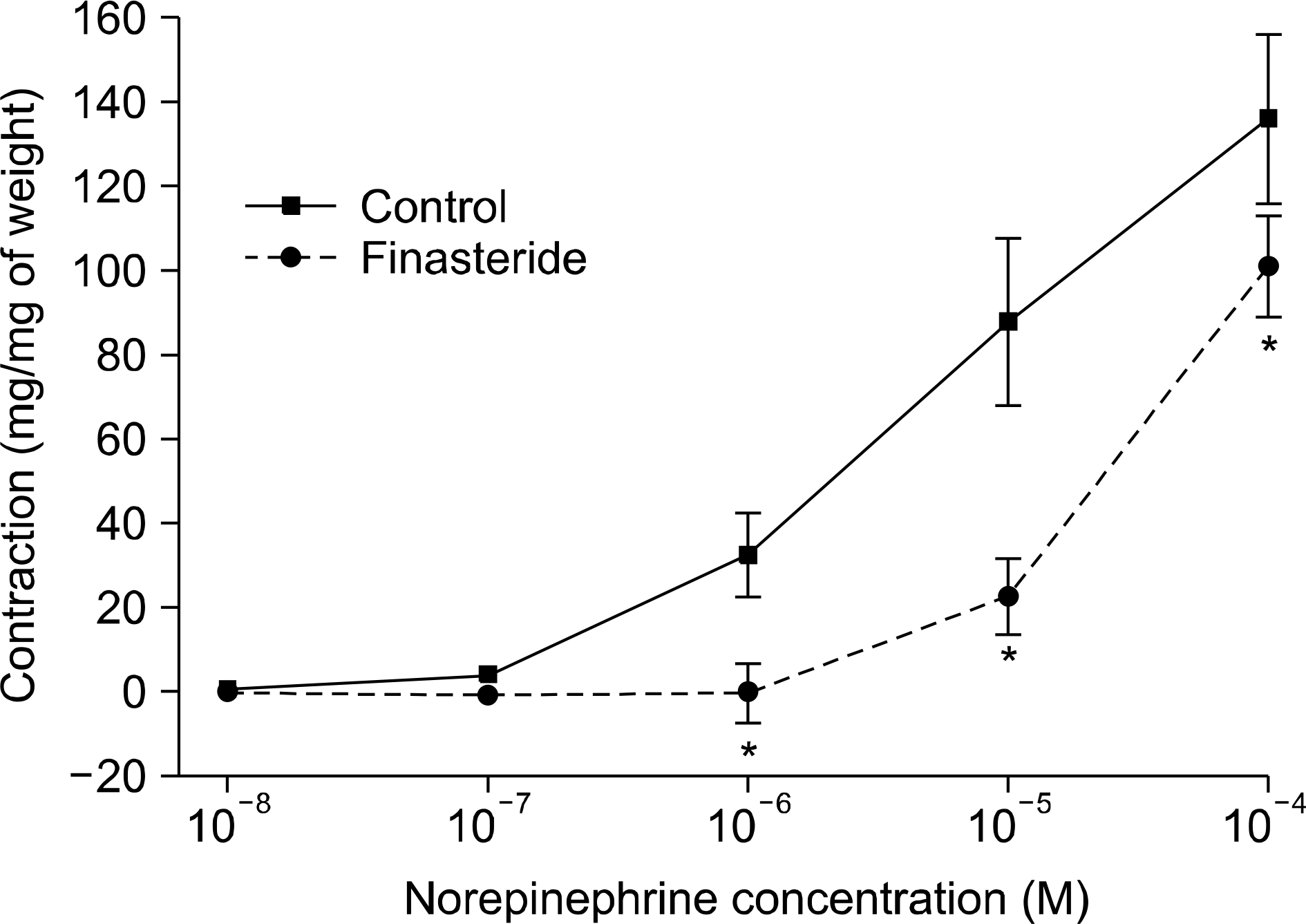 | Fig. 3.Comparison for the effect of finasteride medication on rabbit seminal vesicle strips contraction in norepinephrine dose-dependent manner (n=12, Asterisk indicates p-values are <0.05). Error bars indicate mean±standard error. |
Table 1.
Comparison of semen parameter according to finasteride medication




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download