Abstract
Purpose
The aim of this study was to evaluate Cyanidin-3-O-β-d-glucopyranoside on improvement and protection for erectile function.
Materials and Methods
Sprague-Dawley rats (12wks old) were divided into three groups (n=12 in each): normal control, diabetes (DM), and diabetes with Cyanidin-3-O-β-d-glucopyranoside (C3G) concentration materials treatment (DM+C3G). DM and DM+C3G group received a single injection of streptozotocin (50 mg/kg), and 4 wk after induction of diabetes, DM+C3G group were treated with daily C3G (10 mg/kg) dissolved in water for 8 wk. After 12 wk of streptozotocin injections, rats in each group underwent intracavernosal pressure measurement (ICP) and then the corporal tissues were sampled.
Go to : 
REFERENCES
2). Fedele D, Bortolotti A, Coscelli C, Santeusanio F, Chatenoud L, Colli E, et al. Erectile dysfunction in type 1 and type 2 diabetics in Italy. On behalf of Gruppo Italiano Studio Deficit Erettile nei Diabetici. Int J Epidemiol. 2000; 29:524–31.
3). Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000; 163:460–3.

4). Blanco R, Saenz de Tejada I, Goldstein I, Krane RJ, Wotiz HH, Cohen RA. Dysfunctional penile cholinergic nerves in diabetic impotent men. J Urol. 1990; 144:278–80.

5). Pegge NC, Twomey AM, Vaughton K, Gravenor MB, Ramsey MW, Price DE. The role of endothelial dysfunction in the pathophysiology of erectile dysfunction in diabetes and in determining response to treatment. Diabet Med. 2006; 23:873–8.

6). Jeremy JY, Jones RA, Koupparis AJ, Hotston M, Persad R, Angelini GD, et al. Reactive oxygen species and erectile dysfunction: possible role of NADPH oxidase. Int J Impot Res. 2007; 19:265–80.

7). Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y, et al. Polyhydroxylated alkaloids isolated from mulberry trees (Morusalba L.) and silkworms (Bombyx mori L.). J Agric Food Chem. 2001; 49:4208–13.
8). Dugo P, Mondello L, Errante G, Zappia G, Dugo G. Identification of anthocyanins in berries by narrow-bore high-performance liquid chromatography with electrospray ionization detection. J Agric Food Chem. 2001; 49:3987–92.

9). Mitcheva M, Astroug H, Drenska D, Popov A, Kassarova M. Biochemical and morphological studies on the effects of anthocyans and vitamin E on carbon tetrachloride induced liver injury. Cell Mol Biol (Noisy-le-grand). 1993; 39:443–8.
10). Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001; 8:362–9.

11). Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, et al. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003; 73:1097–114.

12). Kim HB, Kim SL, Koh SH, Seok YS, Kim YS, Sung GB, et al. The deelopmentv of natural pigment with mulberry fruit as a food additive. Korean J Crop Sci. 2011; 56:18–22.
13). Becker AJ, Uckert S, Stief CG, Scheller F, Knapp WH, Machtens SA, et al. Systemic and cavernous plasma levels of vasoactive intestinal polypeptide during sexual arousal in healthy males. World J Urol. 2002; 20:59–63.

14). Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987; 327:524–6.

15). Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992; 326:90–4.

16). Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–30.

17). Keegan A, Cotter MA, Cameron NE. Effects of chelator treatment on aorta and corpus cavernosum from diabetic rats. Free Radic Biol Med. 1999; 27:536–43.

18). Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008; 28:5106–19.
19). Evgenov OV, Liaudet L. Role of nitrosative stress and activation of poly (ADP-ribose) polymerase-1 in cardiovascular failure associated with septic and hemorrhagic shock. Curr Vasc Pharmacol. 2005; 3:293–9.
20). Akingba AG, Burnett AL. Endothelial nitric oxide synthase protein expression, localization, and activity in the penis of the alloxan-induced diabetic rat. Mol Urol. 2001; 5:189–97.

21). Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003; 52:2353–62.

22). Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006; 391:122–6.
Go to : 
 | Fig. 1.Masson's trichrome staining for collagen (blue) and smooth muscle (red) in corporal tissue of the control (A), diabetes (B), and diabetes treated with C3G (C) groups. ×200. |
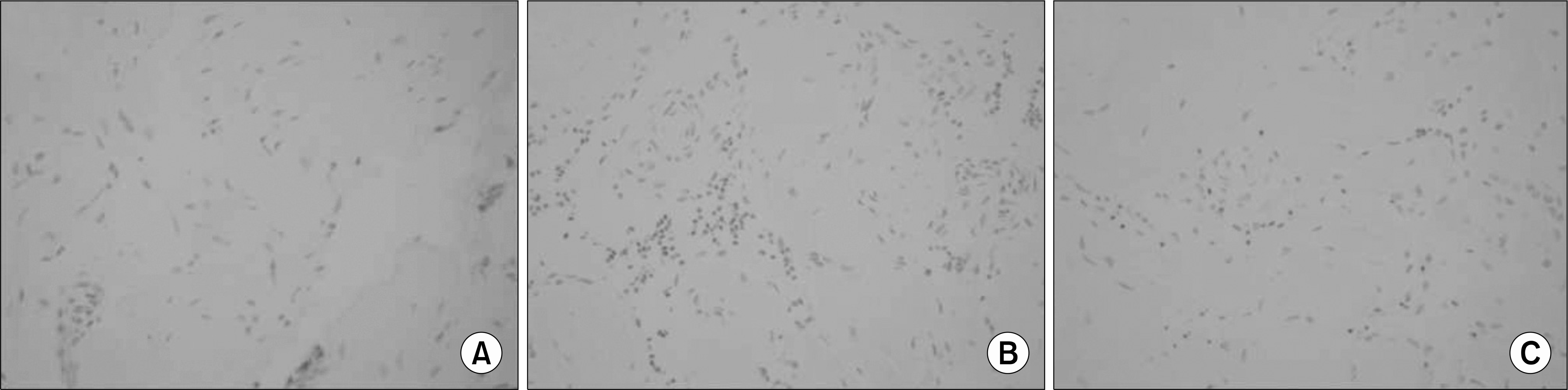 | Fig. 2.Immunohistochemical in situ TUNEL detection of apoptosis in corporal tissue of the control (A), diabetes (B), and diabetes treated with C3G (C) groups. Cells undergoing apoptosis, called apoptotic bodies, show as black or dark brown in the TUNEL assay, while living cells are shown as lighter dots. ×400. |
Table 1.
Changes in body weight and serum glucose levels in the experimental groups
Table 2.
Intracavernosal pressure in response to electrical stimulation of the cavernous nerve in rats from each experimental group
| Control | DM | DM+C3G | p-value | |
|---|---|---|---|---|
| Peak ICP | 83.3±1.9 | 35.4±4.5 | 58.0±4.6 |
0.023∗
0.039† 0.004‡ |
| MAP | 108.5±3.4 | 109.4±2.8 | 104.6±4.5 | |
| ICP/MAP ratio | 0.77±0.05 | 0.32±0.06 | 0.55±0.11 |
0.031∗
<0.001† 0.023‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download