Abstract
Recent advances in laser technology have provided a varied arsenal for endoscopic treatment of benign prostatic hyperplasia. Laser is a collimated coherent radiation of photons generated by stimulated emission of gain media, allowing transfer of selective, controlled and focused energy to the targeted tissue. The application of laser to prostate surgery developed hand-in-hand with refinements to the equipment. Earlier lasers were low powered modalities with no significant tissue selectivity, aimed at thermal coagulation and resulted in significant side effects and recurrence. Since then, prostate lasers have developed towards a more high-powered and selective modality that allowed complete ablation of the tissue with fewer complications. Fiber technology has also developed to allow efficient and safe transfer of a continuously increasing energy output. It is important for the surgeon to understand these fundamental principles of laser and prostate surgery, not only to select the proper tools, but also to properly implement the technique as well.
Go to : 
REFERENCES
1). Costello AJ, Bowsher WG, Bolton DM, Braslis KG, Burt J. Laser ablation of the prostate in patients with benign prostatic hypertrophy. Br J Urol. 1992; 69:603–8.

2). Yariv A, Kwong SK. Theory of laser oscillation in resonators with photorefractive gain. Opt Lett. 1985; 10:454–6.

3). Yariv A. Energy and power considerations in injection and optically pumped lasers. P IEEE. 1963; 51:1723–31.

4). Chen Y. Passive Q-switching of an intracavity frequency doubled diode-pumped Nd: YVO/sub 4//KTP green laser with Cr/sup 4+: YAG. Phot Tech Lett IEEE. 1997; 9:1481–3.
5). Niemz MH. Laser-tissue interactions: fundamentals and applications. 3 ed.Germany: Springer Verlag;2004. p. 15–8.
6). Bachmann A, Ruszat R. The KTP-(greenlight-) laser–principles and experiences. Minim Invasive Ther Allied Technol. 2007; 16:5–10.
7). Sountoulides P, Tsakiris P. The evolution of KTP laser vaporization of the prostate. Yonsei Med J. 2008; 49:189–99.

8). Gilling PJ, Kennett K, Das AK, Thompson D, Fraundorfer MR. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: an update on the early clinical experience. J Endourol. 1998; 12:457–9.

9). Laguna MP, Alivizatos G, De La Rosette JJ. Interstitial laser coagulation treatment of benign prostatic hyperplasia: is it to be recommended? J Endourol. 2003; 17:595–600.

10). Gilling PJ, Cass CB, Cresswell MD, Malcolm AR, Fraundorfer MR. The use of the holmium laser in the treatment of benign prostatic hyperplasia. J Endourol. 1996; 10:459–61.

11). Gilling PJ, Cass CB, Malcolm AR, Fraundorfer MR. Combination holmium and Nd: YAG laser ablation of the prostate: initial clinical experience. J Endourol. 1995; 9:151–3.
12). Cowles RS 3rd, Kabalin JN, Childs S, Lepor H, Dixon C, Stein B, et al. A prospective randomized comparison of transurethral resection to visual laser ablation of the prostate for the treatment of benign prostatic hyperplasia. Urology. 1995; 46:155–60.

13). Hoffman RM, MacDonald R, Slaton JW, Wilt TJ. Laser prostatectomy versus transurethral resection for treating benign prostatic obstruction: a systematic review. J Urol. 2003; 169:210–5.

14). Oesterling JE. Benign prostatic hyperplasia. Medical and minimally invasive treatment options. N Engl J Med. 1995; 332:99–109.
16). Malek RS, Barrett DM, Kuntzman RS. High-power potassium-titanyl-phosphate (KTP/532) laser vaporization prostatectomy: 24 hours later. Urology. 1998; 51:254–6.

17). Paschotta R, Fiedler K, Kurz P, Mlynek J. Nonlinear mode coupling in doubly resonant frequency doublers. Appl Phys B-Lasers O. 1994; 58:117–22.

18). Kuntzman RS, Malek RS, Barrett DM, Bostwick DG. Potassium-titanyl-phosphate laser vaporization of the prostate: a comparative functional and pathologic study in canines. Urology. 1996; 48:575–83.

19). Kang HW, Jebens D, Malek RS, Mitchell G, Koullick E. Laser vaporization of bovine prostate: a quantitative comparison of potassium-titanyl-phosphate and lithium triborate lasers. J Urol. 2008; 180:2675–80.

21). Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on longterm outcomes. J Urol. 2005; 174:1344–8.

22). Hai MA, Malek RS. Photoselective vaporization of the prostate: initial experience with a new 80 W KTP laser for the treatment of benign prostatic hyperplasia. J Endourol. 2003; 17:93–6.

23). Sandhu JS, Ng C, Vanderbrink BA, Egan C, Kaplan SA, Te AE. High-power potassium-titanyl-phosphate photoselective laser vaporization of prostate for treatment of benign prostatic hyperplasia in men with large prostates. Urology. 2004; 64:1155–9.

24). Ruszat R, Seitz M, Wyler SF, Abe C, Rieken M, Reich O, et al. GreenLight laser vaporization of the prostate: single-center experience and longterm results after 500 procedures. Eur Urol. 2008; 54:893–901.

25). Sarica K, Alkan E, Lüleci H, Taşci AI. Photoselective vaporization of the enlarged prostate with KTP laser: longterm results in 240 patients. J Endourol. 2005; 19:1199–202.

26). Sulser T, Reich O, Wyler S, Ruszat R, Casella R, Hofstetter A, et al. Photoselective KTP laser vaporization of the prostate: first experiences with 65 procedures. J Endourol. 2004; 18:976–81.

27). Borboroglu PG, Kane CJ, Ward JF, Roberts JL, Sands JP. Immediate and postoperative complications of transurethral prostatectomy in the 1990s. J Urol. 1999; 162:1307–10.

28). Heinrich E, Wendt-Nordahl G, Honeck P, Alken P, Knoll T, Michel MS, et al. 120 W lithium triborate laser for photoselective vaporization of the prostate: comparison with 80 W potassium-titanyl-phosphate laser in an ex-vivo model. J Endourol. 2010; 24:75–9.
30). Nishioka NS, Domankevitz Y, Flotte TJ, Anderson RR. Ablation of rabbit liver, stomach, and colon with a pulsed holmium laser. Gastroenterology. 1989; 96:831–7.

31). van Leeuwen TG, van der Veen MJ, Verdaasdonk RM, Borst C. Noncontact tissue ablation by holmium: YSGG laser pulses in blood. Lasers Surg Med. 1991; 11:26–34.

32). Gilling PJ, Cass CB, Cresswell MD, Fraundorfer MR. Holmium laser resection of the prostate: preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology. 1996; 47:48–51.

33). Woods E. Laser ablation of the prostate: a safe effective treatment of obstructive benign prostatic disease. Can Urol Assoc J. 2010; 4:344–6.

34). Fried NM, Murray KE. High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol. 2005; 19:25–31.
36). Wendt-Nordahl G, Huckele S, Honeck P, Alken P, Knoll T, Michel MS, et al. Systematic evaluation of a recently introduced 2-microm continuous-wave thulium laser for vaporesection of the prostate. J Endourol. 2008; 22:1041–5.
37). Chiang PH, Chen CH. Prostate vaporization in the treatment of benign prostatic hyperplasia by using a 200-w high-intensity diode laser. Curr Urol Rep. 2010; 11:249–53.

38). Hill KO, Fujii Y, Johnson DC, Kawasaki BS. Photosensitivity in optical fiber waveguides: application to reflection filter fabrication. Appl Phys Lett. 1978; 32:647–9.

39). Nazif OA, Teichman JM, Glickman RD, Welch AJ. Review of laser fibers: a practical guide for urologists. J Endourol. 2004; 18:818–29.

40). Vassar GJ, Teichman JM, Glickman RD. Holmium: YAG lithotripsy efficiency varies with energy density. J Urol. 1998; 160:471–6.

41). Peng SY, Kang HW, Pirzadeh H, Stinson D. MoXy fiber with active cooling cap for bovine prostate vaporization with high power 200W 532 nm laser. Proc SPIE. 2011. 7883ID.

42). Jansen ED, van Leeuwen TG, Motamedi M, Borst C, Welch AJ. Temperature dependence of the absorption coefficient of water for midinfrared laser radiation. Lasers Surg Med. 1994; 14:258–68.

43). Gilling PJ, Cass CB, Malcolm A, Cresswell M, Fraundorfer MR, Kabalin JN. Holmium laser resection of the prostate versus neodymium: yttrium-aluminum-garnet visual laser ablation of the prostate: a randomized prospective comparison of two techniques for laser prostatectomy. Urology. 1998; 51:573–7.
44). Te AE. The Next Generation in Laser Treatments and the Role of the GreenLight High-Performance System Laser. Rev Urol. 2006; 8(Suppl 3):S24–30.
Go to : 
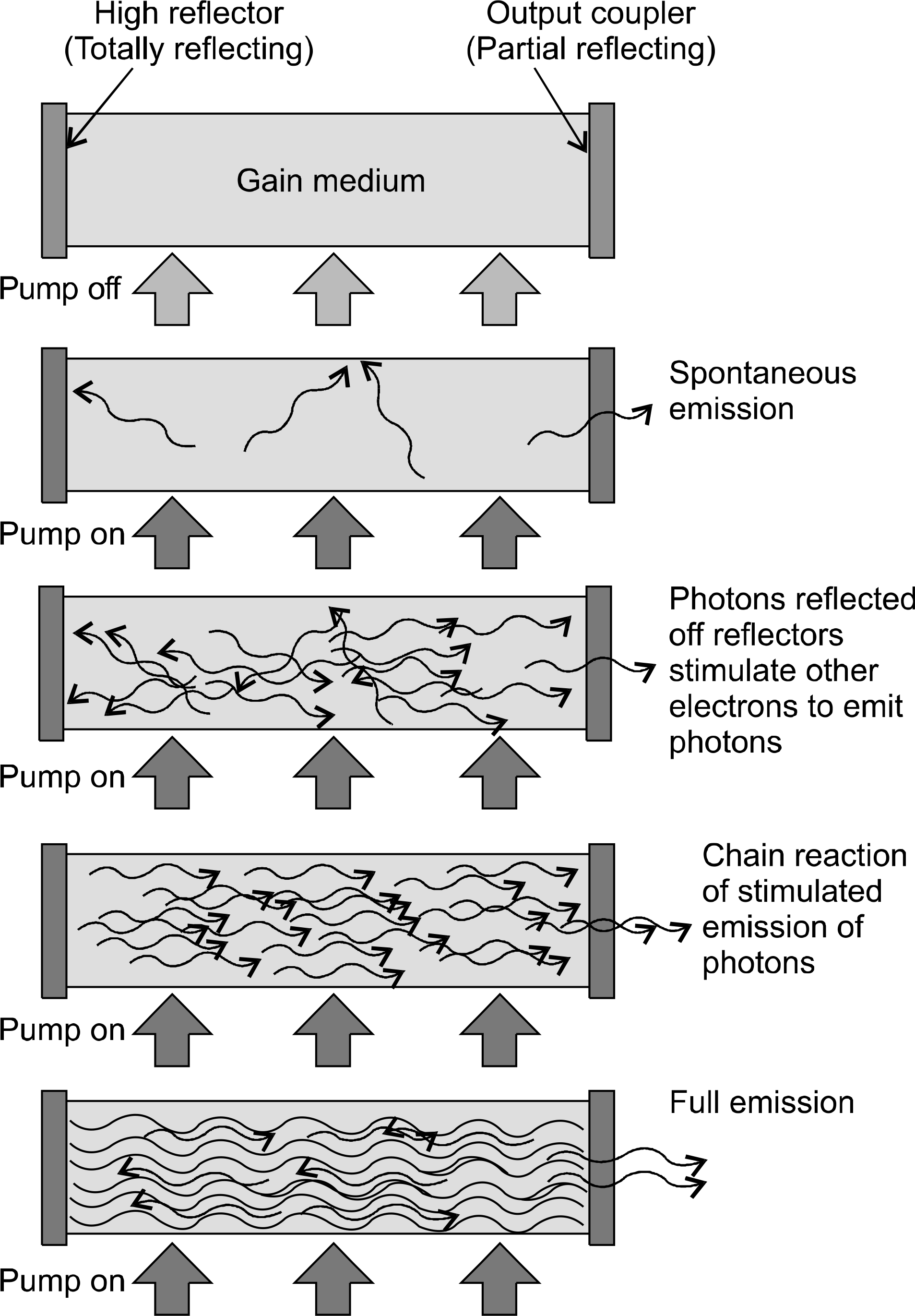 | Fig. 1.Lasers are generated by stimulated emission, brought about by pumping the gain medium to an excited state. Released photons oscillate within the optical resonator to emit a collimated, coherent monochromatic electromagnetic radiation. |
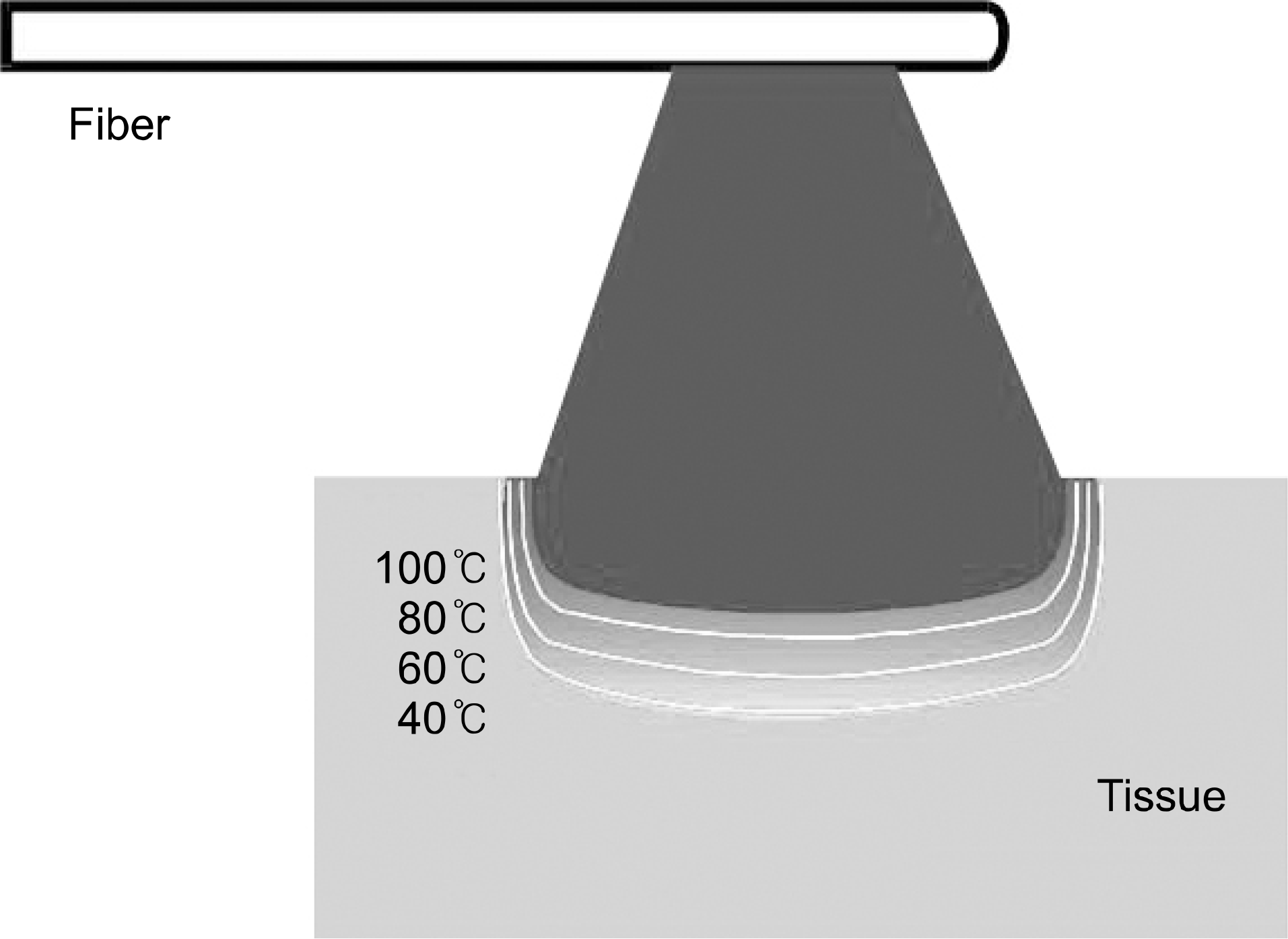 | Fig. 2.Tissue effect can also vary with the depth of penetration, due to different levels of temperature achieved within the affected zone.42
|
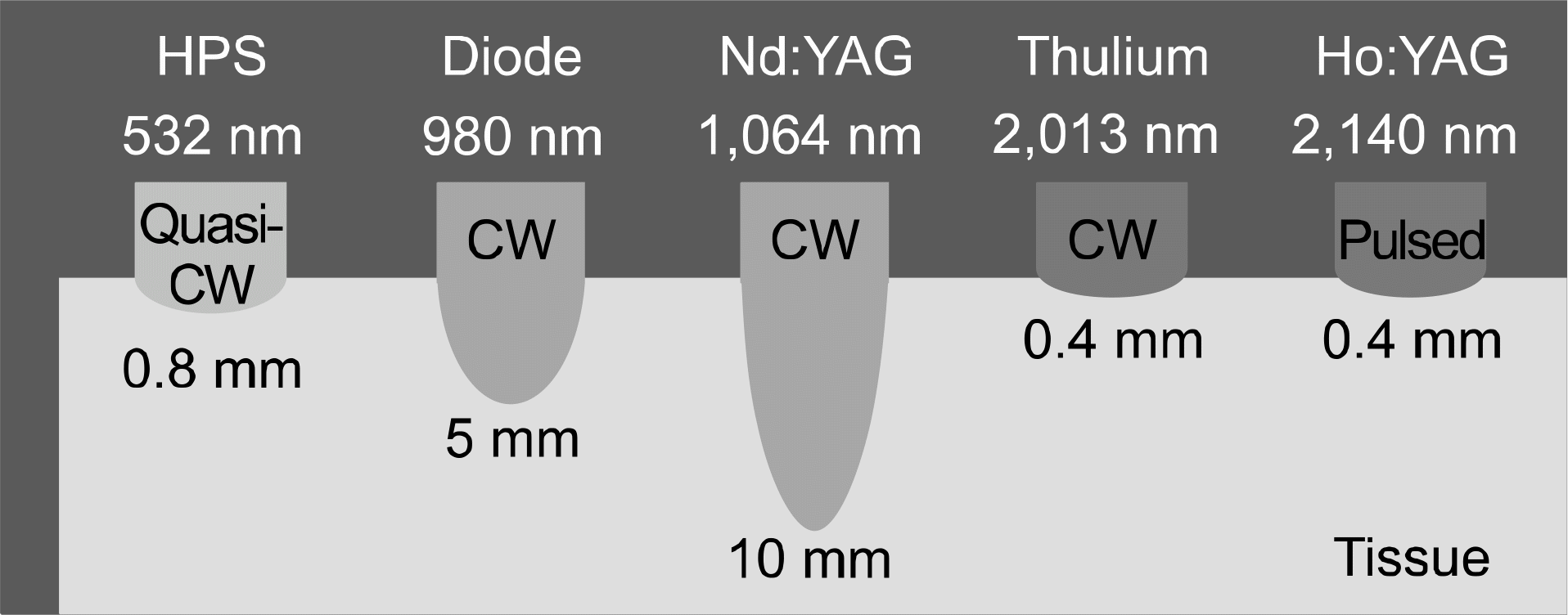 | Fig. 3.Laser wavelength and mode of emission can affect the depth of penetration, leading to a varied profile of energy density (HPS: high performance system, CW: continuous wave).42
|
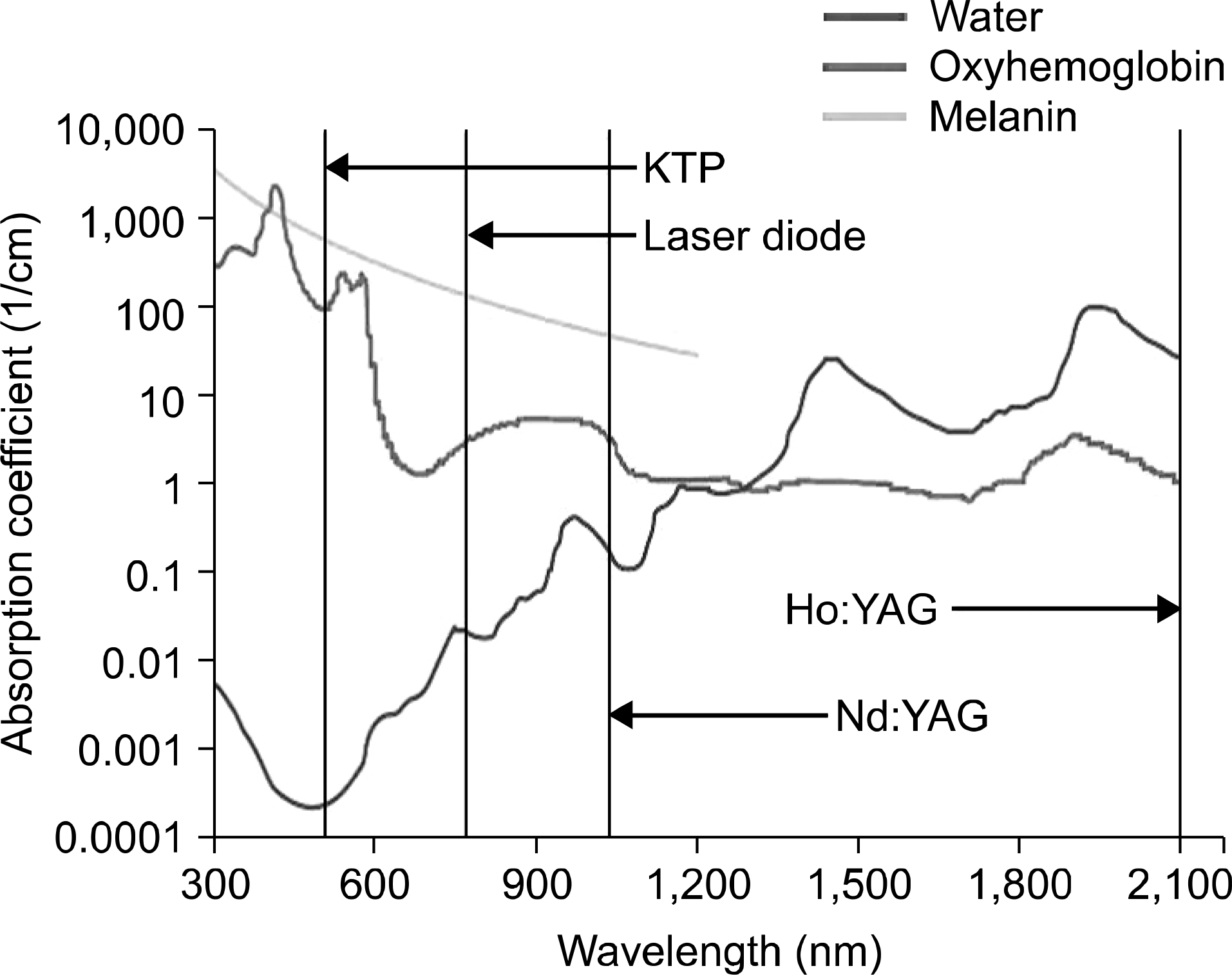 | Fig. 4.Chromophores allow selective absorption of specific wavelengths, vastly improving the tissue effect of certain lasers. Early Nd:YAG lasers are generally nonspecific to either water or hemoglobin. Modern green light lasers are highly absorbed by hemoglobin, while modern infrared lasers are highly absorbed by water.5
|
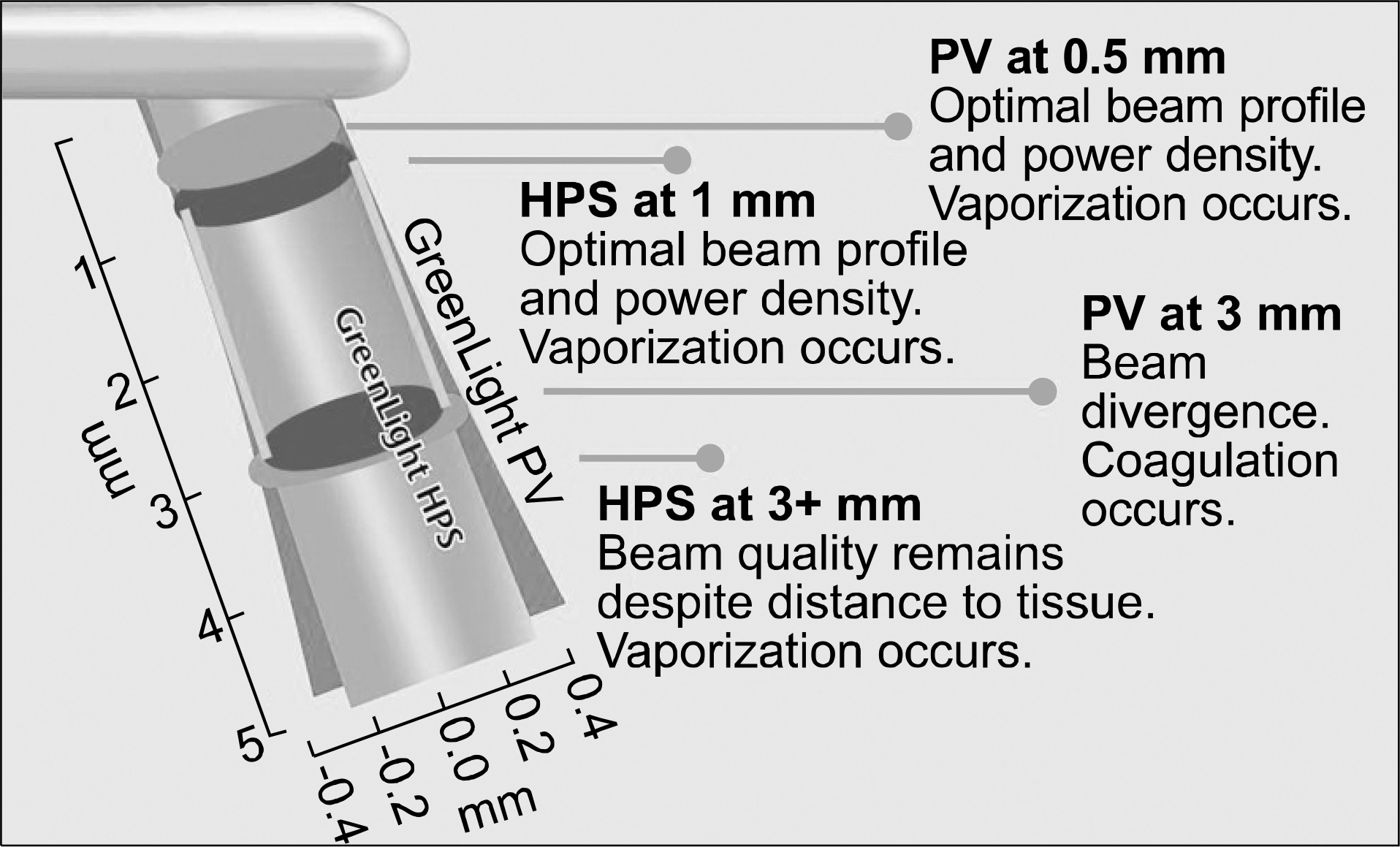 | Fig. 5.Increased power allows the beam to be more collimated. The tissue laser interaction not only benefits from more power, but also from a more focused high intensity transfer of energy (HPS: high performance system, PV: photo vaporization).44
|
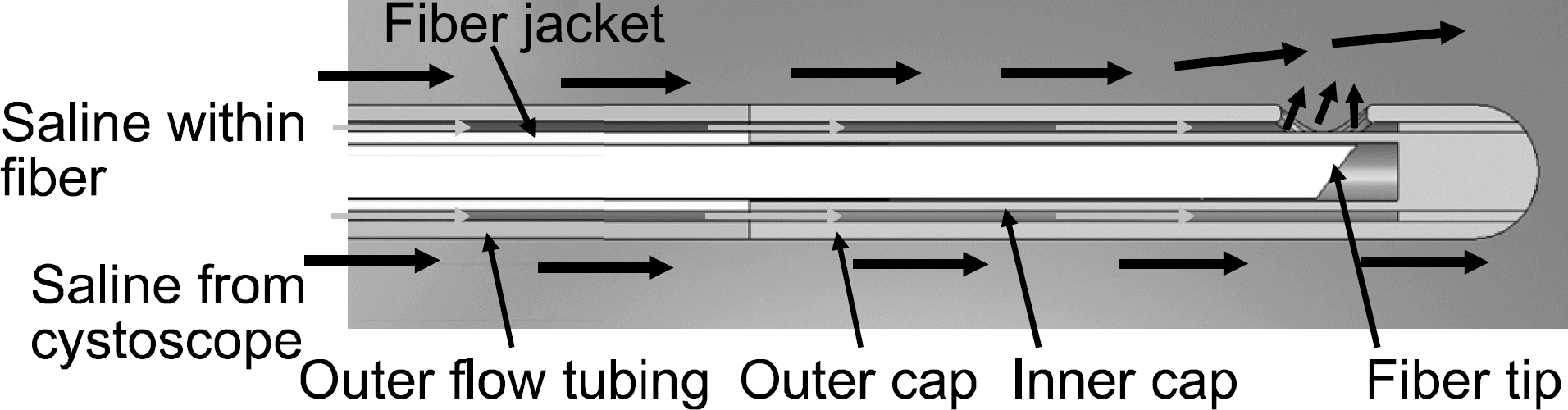 | Fig. 6.Laser fibers also develop to accommodate the increased laser energy. Newer fibers are designed to allow more flexibility, less loss of energy and greater safety.41
|
Table 1.
Different tissue effects appear by level of energy transfer42
| <40oC | Photobiomodulation |
|---|---|
| 42∼45oC 45∼50oC | Hyperthermia Desiccation |
| 50∼100oC | Coagulation, irreversible |
| >60oC | Protein denaturation |
| >100oC | Carbonization, vaporization |
Table 2.
Prostate laser developed from the early low power Nd:YAG to the contemporary modalities through increased power and optimized wavelengths29,37,43,44
VLAP: visual laser ablation of the prostate, CELAP: combined endoscopic laser ablation of the prostate, ILC: interstitial laser coagulation, HoLAP: holmium laser ablation of the prostate, HoLRP: holmium laser resection of the prostate, HoLEP: holmium laser enucleation of the prostate, PVP: photovaporization of the prostate, HPS: high performance system.
Table 3.
Different lasers are characterized primarily by wavelengths. However, interactions with different chromophores, and modes of generation also affect tissue interactions and depth of tissue effect10,20,37




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download