Abstract
Background
The use of natural bioactive compounds in conventional chemotherapy is a new direction in cancer treatment that is gaining more research attention recently. Bioactive polysaccharides and polysaccharide-protein complexes from some fungi (edible mushrooms) have been identified as sources of effective and non-toxic antineoplastic agents. Selected oyster mushrooms (Pleurotus pulmonarius and P. ostreatus being local [Nigeria] and exotic strains, respectively) were cultured on a novel medium of yeast extract supplemented with an ethanolic extract of Annona senegalensis, and the antileukemic potential of their metabolites was studied.
Methods
Leukemia was successfully induced in Wister rats by intravenous injection (0.2 mL) of a benzene solution every 2 days for 3 consecutive weeks. The aqueous solution of fungal metabolites (20 mg/mL) produced by submerged fermentation was orally administered (0.2 mL) before, during, and after leukemia induction. Leukemia burden was assessed by comparing the hematological parameters at baseline and after leukemia induction. The immunomodulatory potential of the metabolites was assessed by using a phagocytic assay (carbon clearance method). The ability to enhance leukopoiesis was assessed by using the total leukocyte count.
The global awareness of leukemia as one of the major causes of cancer death worldwide in people of various ages and racial background has led to intensive research efforts and numerous clinical studies in the fight against the disease [1]. Leukemia, a cancer of the blood or bone marrow, is characterized by an abnormal increase in blood cells, usually leukocytes, which results from somatic mutations in the DNA. Certain mutations produce leukemia by activating oncogenes or deactivating tumor suppressor genes, thereby disrupting the regulation of cell death, differentiation, or division. These mutations may occur spontaneously or after exposure to radiation or carcinogenic substances and are likely to be influenced by genetic factors [2].
Currently, researchers are focusing on organisms that appear to offer anti-cancer and immune system enhancing activity. Depending on the stage of cancer progression, treatments include surgical operation, radiotherapy, and chemotherapy. Most cancer chemotherapies are essentially toxic, because it is difficult to apply the principle of selective toxicity used in the treatment of microbial infections. Patients receiving these agents experience severe side effects that limit the doses which can be administered, and hence, limit the beneficial effects. Clinical investigators realized that the ability to manage these toxicities is crucial to the success of cancer chemotherapy. However, their side effects cause serious damage and negatively affect the patients. As an alternative to these treatment methods, immunotherapy is now gaining more attention than ever. Immunotherapy substantially reduces the side effects and the inherent pain of cancer experienced by patients and helps to overcome cancer growth, even in the last stages of the disease [3].
Recent investigations have been channeled on the development of immunotherapies to target and remove cancer cells as well as on substances such as immunopotentiators, immunoinitiators, and biological response modulators that act to prevent carcinogenesis and induce carcinostasis [1].
Some polysaccharides or polysaccharide-protein complexes from mushrooms (fungi) are able to stimulate the non-specific immune system and exert antitumor activities through stimulation of the host's defense mechanism [4]. These substances activate effector cells such as macrophages, T lymphocytes, and natural killer (NK) cells to secrete cytokines, e.g., tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β, etc., which are anti-proliferative and induce apoptosis and differentiation in tumor cells. Pleurotus ostreatus and P. pulmonarius are edible mushrooms possessing biological activities related to immune enhancement and in vitro anticancer effects [3].
This work aimed at determining the antileukemic action of metabolites from P. pulmonarius, a local strain, and P. ostreatus, an exotic strain, and comparing their potentials.
P. pulmonarius and P. ostreatus metabolites were produced by submerged fermentation at the Biotechnology Laboratory of Ladoke Akintola University of Technology, Ogbomoso, Nigeria. P. pulmonarius and P. ostreatus were cultured/fermented in standard yeast extract-glucose medium supplemented with 5 g/L ethanolic extract of Annona senegalensis. The yeast extract-glucose medium consisted of: glucose 50 g/L, peptone 2.5 g/L, yeast extract 2.5 g/L, KH2PO4 2 g/L, MgSO4.7H2O 1 g/L, and CaCl2.2H2O 1 g/L; the pH was maintained at 5.8. The fungi were inoculated into separate fermentation flasks by using 3 agar plugs each (6 mm diameter) from the outer circumference of a fungal colony growing on a potato dextrose agar medium plate. This served as the inoculum [5]. The media were then incubated on an orbital shaker at 28℃ for 7 days at 150 rpm. The medium was harvested after 7 days of fermentation by using a cheesecloth to sieve in order to obtain the mycelia mass. The filtrate was measured by using a measuring cylinder and an equal volume of acetone was added to precipitate the metabolites. The broth was filtered with filter paper to remove the metabolites. The concentration of the metabolite solutions was adjusted to 20 mg/mL.
The rats were administered 0.2 mL of the metabolite solution by gavage daily before, during, and after leukemia induction as designed in the experimental protocol (Table 1).
Ninety-six Wister rats weighing about 150 g each were purchased from the animal house of Obafemi Awolowo University, Ile-Ife, Nigeria. The rats were randomly arranged in separate wooden cages (16 per group; 2 replicates of 8 each) and were allowed to acclimatize for 7 days before commencement of the experiment. The room temperature in the animal house was 28±2℃ and a 12-hr light/dark cycle was employed. The rats were handled following standard ethical procedures.
The commercial (control) diet for the rats was purchased from the animal house of the Ladoke Akintola University of Technology, Osogbo, Nigeria. The rats were fed rat pellets and water ad libitum.
Leukemia was successfully induced in Wister rats by intravenous injection of 0.2 mL of a 1:10 diluted benzene solution (Chromasolv, in water/2-propanol [50/50] v/v), given every 2 days for 3 consecutive weeks. The aqueous solution of the mushroom metabolites (20 mg/mL) produced by submerged fermentation was orally administered by gavage (0.2 mL) before, during, and after leukemia induction. Leukemia burden was assessed by comparing the hematological parameters at baseline and after leukemia induction in various experimental groups (Table 1).
After 3 weeks of benzene injection and administration of metabolites (as designed in the experimental protocol), animals in the respective groups were bled by cardiac puncture. The blood was collected into ethylenediaminetetraacetic acid (EDTA) vials, gently mixed, labeled, and analyzed. The animals were handled and killed ethically as recommended by animal care regulations.
Hematological parameters and indices were assessed by flow cytometry (direct current method) using suitable cell packs according to the manufacturer's specification for the desired cell population on the SYMEX KX-21N autoanalyzer.
The Leishman staining technique was used to compliment the automation estimation result. The technique was applied as previously described [6]. The stained slides were allowed to dry and examined using the battlement method with an oil immersion objective (×100). One hundred consecutive leukocytes indicating various types encountered were counted and recorded.
Leukocytes comprise of macrophages and NK cells have been shown to play important roles in cancer surveillance in the human body and subsequently elimination of cancerous cells [3]. In order to determine the immunomodulatory functions of the metabolites, their potential to enhance bone marrow leukopoiesis was determined on the basis of the total leukocyte count and phagocytic function of WBCs as measured using the carbon clearance test described below.
The rats were divided into 4 groups of 8 animals in each group, received various treatments as mentioned below, and were used for the above-described assays.
The characteristics of the groups were as follows: group 1: control (normal) rats were given only normal saline, while rats in groups 2, 3, and 4 received doses of 20 mg/mL, 40 mg/mL, and 80 mg/mL, respectively. All solutions were administered using an oral cannula, once per day for 8 days.
The leukocytes were counted as previously described [7]. Blood samples were collected into EDTA anticoagulant vials through the tail of Wister rats on the 8th day of metabolite administration. The blood (20 µL) was diluted in 0.38 mL of Turk's solution and the suspension was charged onto the new improved Neubauer counting chamber. The cells were counted microscopically using a 16-mm eyepiece and a ×10 objective.
The phagocytic activity of the reticuloendothelial system (RES) was assessed by using the carbon clearance method [8]. Briefly, 1 mL of Indian ink was administered intravenously to all 4 groups of adult Wister rats on the 8th day of metabolite administration. Blood samples were collected at 3-, 6-, 9-, and 12-min intervals and transferred directly into the centrifuge tube, allowed to coagulate at room temperature, and centrifuged at 2,000 rpm for 10 min. Then, 50 µL of clear supernatant (serum) was collected and transferred to different volumetric flask. The volume was made up to 25 mL using distilled water; absorbance was measured at 650 nm using a spectrophotometer. The recorded absorbance was plotted against time. The slope of the graph denotes the rate of carbon clearance, which is a measure of phagocytic activity of the RES and termed phagocytic index [9].
Results of the hematological parameters (which are the major leukemia indices) in the various experimental groups treated with P. pulmonarius and P. ostreatus metabolites are shown in Tables 2 and 3 and Figs. 1 and 2, respectively. The Tables and Figs show the statistical comparisons of various hematological parameters between baseline and post-treatment of the respective experimental groups.
Group A showed a slight decrease in packed cell volume (PCV) when the baseline results were compared with their respective post-treatment values. PCV is a measure of the volume of RBCs compared to the total volume of blood and is a reliable indication of the amount of circulating RBCs and the degree of anemia or polycythemia in the experimental rats. A similar decrease was observed in the PCV of groups B and C, when their baseline and post-treatment values were compared (Tables 2 and 3, respectively).
Groups D and F were found to have a normal PCV. Results of various hematological parameters in group D revealed that there was no adverse reaction or toxicity experienced by the rats as a result of administration of the 2 metabolites.
Groups A, B, C, and D were found to have a slight and insignificant increase in WBC counts, when their baseline values were compared with their post-treatment values, but WBC count in group E markedly increased, when the baseline leukocyte counts were compared with the post-treatment counts, which was an indication of successfully-induced leukemia.
The erythrocyte counts in groups A and C were found to have increased, when their baseline value were compared with the post-treatment values. A similar increase was observed in the PCV of group D showing non-toxicity of the metabolites. The healthy animals treated with P. ostreatus and P. pulmonarius metabolites presented in group D showed no significant alterations in erythrocyte counts, leukocyte counts, and hematological indices, when compared with the healthy animals in group F who received commercial feed and water only.
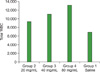
The total WBC (TWBC) counts in the control group served as reference values. In animals treated with P. ostreatus and P. pulmonarius metabolites, the number of leukocytes increased. The highest count was obtained from the animal group fed with metabolites at the highest dose (80 mg/mL) (P<0.001) (Fig. 3 and 4). There was a statistically significant increase (P<0.05) in the leukocyte counts among the treatment groups (Table 4).
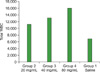
The results presented in Figs. 3 and 4 (P. ostreatus and P. pulmonarius, respectively) show that the concentration of carbon ink obtained after 12 min of experimental time decreased compared with the initially recorded value (at 3 min). Comparatively, P. pulmonarius metabolites with mean phagocytic indices of 2.26, 2.03, and 1.97, respectively, demonstrated a significantly better phagocytic activity than P. ostreatus (P<0.05), as shown in Table 5.
In this study, anemia of chronic disease was observed in animals of groups A, B, and C where leukemia was induced due to exposure to a potent carcinogen [10]. A significant increase was observed in the hemoglobin concentration and number of erythrocytes in groups A, B, and C, when compared with the leukemia-positive group (Fig. 1 and 2). This observation suggests that the metabolites of P. pulmonarius and P. ostreatus have beneficial effects on anemia in animals with leukemia. This finding is supported by the report on the hematological and metabolic effects of dietary supplementation with the fungus Agaricus sylvaticus on rats bearing solid Walker 256 tumors [10].
Leukocytosis was observed in the groups of leukemia-induced rats as demonstrated in some other works [11, 12]. There was a significant difference in WBCs between group C and group E. Leukocytosis is usually associated with leukemia and was found to be restored or prevented in rats treated with either of the metabolites before, during, or after exposure to the leukemia-inducing agent because there was no significant difference in the post-treatment WBC counts of groups A, B, and C. This was an indication of the prophylactic potentials of the metabolites (Table 2 and 3).
The result was corroborated by the report of a previous work when the metabolites from the same organisms exhibited profound antitumor activities on N-nitroso-N-ethylurea-induced tumors in experimental animals [13]. The antitumor activity could be attributed to fungal polysaccharide complexes that are capable of stimulating the non-specific immune system and exert antitumor activity through activation of the host's defense mechanism [14].
In this work, we also report the immunomodulatory activity of the investigated metabolites using leukopoiesis enhancement and carbon clearance tests. Findings on leukopoiesis enhancement potential from this study showed that metabolites of P. ostreatus and P. pulmonarius have the ability to increase the total leukocyte count in Wister rats; the highest count was observed in the group that received the highest dose (80 mg/mL). There was a significant difference between the groups (P<0.005) (Table 4). This result is in line with the research carried out by Jain and colleagues [15]. They investigated the immunomodulatory activity of a methanolic extract of Tephrosia purpurea on the 8th day of administration. The extract caused a dose-dependent increase in the leukocyte count at doses of 100, 200, and 400 mL/kg body weight.
The carbon clearance test was employed to evaluate the effect of the fungal metabolites on the RES. The result obtained from this research implies that administration of both metabolites resulted in an increased number and enhanced phagocytic activity of macrophages on the 8th day of administration. The highest values were observed in the group treated with the highest dose (80 mg/mL); thus, the function of the macrophages was significantly enhanced. This was further supported by the significant increase in carbon clearance at a higher dose of the metabolites (80 mg/mL), which indicates stimulation of the RES [16]. However, this result is in contrast to that obtained when the immunomodulatory activity of a polyherbal drug was studied [17]. Mice were fed with the respective drugs orally for 5 days; at 48 h after the last treatment, mice were injected with a carbon ink suspension (0.01 mL) via the tail vein. The result revealed that the rate of clearance of carbon particles was increased by all treatments when compared to the vehicle, but the increase was only significant at a higher dose of the polyherbal formulation (90 mg/kg) and not at a lower dose (45 mg/kg) as well as the standard levamisole (25 mg/kg).
In conclusion, the fungal metabolites investigated in this study exhibited therapeutic potential as demonstrated by their ability to suppress leukemia in animals following oral administration in various experimental groups. The bioactive compounds from both local and exotic mushrooms revealed profound immunotherapeutic and leukemia suppressor activities.
Figures and Tables
Fig. 1
Comparison of hematological parameters after treatment with Pleurotus pulmonarius metabolites between the positive control group (E) and the other treatment groups.
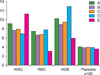
Fig. 2
Comparison of the hematological parameters after treatment with Pleurotus ostreatus metabolites between the positive control group (E) and the other treatment groups.
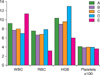
Table 2
Hematological parameters at baseline, post leukemia induction, and after treatment with Pleurotus pulmonarius metabolites (mean±SD).

Table 3
Hematological parameters at baseline, post leukemia induction, and after treatment with Pleurotus metabolites (mean±SD).

ACKNOWLEDGEMENTS
The authors thank the authority of LAUTECH, Ogbomoso, Nigeria, for providing some of the facilities used in these studies. We also appreciate the technical assistance provided by Olaniran O. I. and Adegoke O. of the Pharmacology Department and College animal house, respectively, as well as the support by our research assistants and undergraduate students.
References
1. Wasser SP, Weis AL. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspectives. Int J Med Mushrooms. 1999. 1:31–62.

2. Kasper DL, Braunwald E, Fauci AS, editors. Harrison's principles of internal medicine. 2005. 16th ed. New York, NY: McGraw-Hill;333–339.
3. Petrova RD, Mahajna J, Reznick AZ, Wasser SP, Denchev CM, Nevo E. Fungal substances as modulators of NF-kappaB activation pathway. Mol Biol Rep. 2007. 34:145–154.

4. Reshetnikov SV, Wasser SP, Tan KK. Higher basidiomycota as a source of antitumor and immunostimulating polysaccharides (review). Int J Med Mushrooms. 2001. 3:361–394.

5. Soden DM, Dobson AD. Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology. 2001. 147:1755–1763.

6. Bain BJ, Lewis SM. Lewis SM, Bain BJ, Bates I, editors. Preparation and staining methods for blood and bone marrow films. Practical haematology. 2006. 10th ed. Philadelphia, PA: Churchill Livingstone;59–76.

7. Cheesbrough M, editor. District laboratory practice in Tropical Countries, part 2. 2000. Cambridge low price ed. Cambridge, UK: Cambridge University press;314–315.
8. Hudson L, Hay FC, editors. Practical immunology. 1980. 2nd ed. London, UK: Blackwell Scientific Publications;73.
9. Pallabi D, Dasgupta SC, Gomes A. Immunopotentiating and immunoprophylactic activities of immune-21, a polyherbal product. Indian J Pharmacol. 1998. 30:163–168.
10. Taveira VC, Novaes MR, Dos Anjos Reis M, da Silva MF. Hematologic and metabolic effects of dietary supplementation with Agaricus sylvaticus fungi on rats bearing solid walker 256 tumor. Exp Biol Med (Maywood). 2008. 233:1341–1347.

11. Quemener V, Bansard JY, Delamaire M, et al. Red blood cell polyamines, anaemia and tumour growth in the rat. Eur J Cancer. 1996. 32A:316–321.
12. Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999. 5:487–491.
13. Akanni EO, Oloke JK, Adebayo EA, Ola IO. Antitumour activities of Pleurotus pulmonarius and Pleurotus ostreatus metabolites on N-Nitroso-N-ethylurea induced solid tumour bearing Wister rats. J Pharm Pharm Res. 2010. 1:13–18.
14. A Ajith T, K Janardhanan K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J Clin Biochem Nutr. 2007. 40:157–162.

15. Jain V, Jat RC, Dubey S, Bhardwaj S, Jain S. Immunomodulatory effect of aerial part of Tephrosia purpurea (Linn). J Pharm Res. 2010. 3:156–158.
16. Selvi KS, Suthanthirarajan N, Namasivayam A. Reticuloendothelial function in acute noise stress. Indian J Physiol Pharmacol. 1992. 36:279–281.
17. Razdan R, Roy SM. Study of the immunomodulatory activity of a polyherbal drug. Pharmacologyonline. 2008. 3:336–345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download