Abstract
Background
Granulocyte-colony stimulating factor (G-CSF) is extensively used to improve neutrophil count during anti-cancer chemotherapy. We investigated the effects of G-CSF on several leukemic cell lines and screened for the expression of the G-CSF receptor (G-CSFR) in various malignant cells.
Methods
We examined the effects of the most commonly used commercial forms of G-CSF (glycosylated lenograstim and nonglycosylated filgrastim) on various leukemic cell lines by flow cytometry. Moreover, we screened for the expression of G-CSFR mRNA in 38 solid tumor cell lines by using real-time PCR.
Results
G-CSF stimulated proliferation (40-80% increase in proliferation in treated cells as compared to that in control cells) in 3 leukemic cell lines and induced differentiation of AML1/ETO+ leukemic cells. Among the 38 solid tumor cell lines, 5 cell lines (hepatoblastoma, 2 breast carcinoma, squamous cell carcinoma of the larynx, and melanoma cell lines) showed G-CSFR mRNA expression.
Granulocyte-colony stimulating factor (G-CSF) is extensively used to improve neutrophil count during anticancer chemotherapy [1-3]. In addition to the well-known effect of hematopoietic stem cell mobilization, the roles of G-CSF are diverse: activation of cellular function, inhibition of apoptosis, and increase in cell adhesion [4, 5]. We previously reported increased expression of G-CSFR in AML1/ETO+ AML cells, suggesting that cells with G-CSFR expression may proliferate easily in response to therapeutic G-CSF [2]. In the present study, we investigated the effects of the most commonly used commercial forms of G-CSF (glycosylated lenograstim and nonglycosylated filgrastim) on various leukemic cell lines. Moreover, we screened for the expression of G-CSFR mRNA in 38 solid tumor cell lines using real-time PCR because solid tumors of non-hematopoietic origin have been shown to express G-CSF or G-CSFR [6-13]. Detecting G-CSFR expression in primary tumor cells would allow careful monitoring of patient condition and aid in early detection of proliferation and/or differentiation. To our knowledge, this is the first study providing a quantitative comparison of G-CSFR mRNA expression in various solid tumor cells.
Kasumi or CTV-1 cells, acute myeloid leukemia (AML) cells with or without the AML1/ETO gene rearrangement respectively; K562 chronic myelogenous leukemia (CML) cells; and the U266 multiple myeloma (MM) cell line were obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea) and the America Type Culture Collection (ATCC, Rockville, MD). The cDNA from various solid tumor cell lines including SNU-201, U87MG, and A172, glioblastomas; Hs683, brain glioma; IMR-32, neuroblastoma; A375P, MDA-MB-435, and Malme-3M, melanomas; A498 and 293, renal cell carcinomas; AGS, gastric adenocarcinoma; DU-145, prostate carcinoma; FaDu and SNU-1041, squamous cell carcinomas of the pharynx; HCC-95 and SK-MES-1, squamous cell carcinomas of the lung; HeLa, adenocarcinoma of the cervix; Hep-2, epidermoid carcinoma of the larynx; SNU-899, squamous cell carcinoma of the larynx; HepG2, hepatoblastoma; SNU-368, 423, 449, and 878, hepatocellular carcinomas; OVCAR-3, ovarian adenocarcinoma. SNU-119, ovarian cystadenoma; RPMI2650, nasal squamous cell carcinoma; RT4, transitional cell carcinoma; SNU-410, pancreatic carcinoma; SNU-175 and C2B, carcinoma of colon; UV2237M, fibrosarcoma; MCF-7, MCF-10A, BT20, MDA-MB-231, HCC1954 and T47D, breast carcinomas were kindly provided by the Korean Cell Line Bank (KCLB, Seoul, Korea).
Real-time quantitative PCR was performed using a Universal TaqMan Probe Master Mix (Applied Biosystems Foster City, CA, USA). Amplification was performed at 50℃ for 2 min and 95℃ for 10 min, followed by 40 cycles at 95℃ for 30 sec, 60℃ for 30 sec, and 72℃ for 30 sec. TaqMan analysis was used to detect CSF3R (Hs00167918_m1) and GAPDH (Hs99999905_m1) mRNA expression using primers and conditions designed by assays-on-demand gene expression products (Applied Biosystems, USA). Each of the 384-well real-time quantitative PCR plates contained serial dilutions (1, 1/2, 1/4, 1/8, and 1/16) of cDNA, which were used to generate relative standard curves for CSF3R and GAPDH. The G-CSFR expression was normalized to GAPDH expression. The real-time PCR analysis was performed using an Applied Biosystems Prism 7900 Sequence Detection System (Applied Biosystems, USA). Data were analyzed using ABI Prism 7700 SDS software (version 1.0). The levels of G-CSFR expression were confirmed in 3 independent experiments.
The proliferation of cells was evaluated using a Cell-Titer 96® Non-Radioactive Cell Proliferation Assay (Promega Co., Madison, WI, USA) according to the manufacturer's protocol. Briefly, the cells were suspended to obtain a final concentration of 1×105 cells/mL, and 500 µL of this suspension was incubated at 37℃ for 48-72 h in a humidified, 5% CO2 atmosphere. After 4 h of incubation in a dye solution, 100 µL of solubilization solution/stop mix was added, and the absorbance was recorded at a wavelength of 570 nm. Analysis of cell proliferation using an EdU assay was also performed. A Click-iT™ EdU Alexa Fluor Flow Cytometry Kit (Invitrogen, Eugene, OR, USA) was used in accordance with the manufacturer's instructions. Briefly, G-CSF-treated or untreated Kasumi-1 and CTV-1 cells were incubated with 10 µM EdU in culture media at 37℃ for 60 min. The cells were harvested, fixed, and permeabilized with 5% Triton X-100 for 30 min, and then stained with Alexa Fluor 647 dye in the dark for 30 min. Fluorescence intensity was measured by flow cytometry (BD Biosciences, San Jose, CA), and the percentage of cell proliferation was determined using FlowJo flow cytometry analysis software (Tree Star Inc., Ashland, OR, USA). The results were validated with 2 repeated experiments.
Cell suspensions with the same cell density were placed in sterile culture dishes and treated with 2 forms of G-CSF (filgrastim, lenograstim) at concentrations of 0, 10, 50, and 100 ng/mL for 2 weeks. At 0, 3, 7, and 14 d after G-CSF treatment, cells were harvested and analyzed by triple-staining with fluorescein isothiocyanate, phycoerythrin, and PerCP-conjugated monoclonal antibodies for CD11b and CD66b (Becton Dickinson Biosciences, San Diego, CA, USA and DakoCytomation, Glostrup, Denmark). Negative controls included a mouse isotype-matched non-relevant immunoglobulin. The samples were analyzed by flow cytometry (FACSCanto, Becton Dickinson, Franklin Lakes, NJ, USA). The results were validated by 2 repeated experimentation.
We analyzed G-CSFR expression in Kasumi-1 (AML with AML1/ETO gene rearrangement), CTV-1 (AML without AML1/ETO gene rearrangement), K562 (CML), and U266 cell lines (MM). Kasumi-1 and K562 cells expressed G-CSFR mRNA whereas CTV-1 and U266 did not. Compared to G-CSFR expression in Kasumi-1 cells, K562 expressed a relatively small amount of G-CSFR mRNA (relative expression: 0.02). Among the 38 solid tumor cell lines tested, 5 (13.1%) (hepatoblastoma [HepG2], melanoma [MDA-MB-435], squamous cell carcinoma of larynx [SNU-899], and breast cancer [HCC 1954 and MCF-10A] cell lines) expressed G-CSFR mRNA, with relative G-CSFR expressions of 0.76, 0.03, 0.01, 0.01, and 0.002, respectively. Expression of G-CSFR in the HepG2 cell line was high and comparable to that in the Kasumi-1 cell line (Fig. 1).
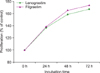
Both forms of G-CSF (filgrastim and lenograstim) significantly and comparably stimulated the proliferation of AML1/ETO+ Kasumi-1 cells at increasing concentrations (10, 50, and 100 ng/mL) (Fig. 2). In K562 cells, treatment with G-CSF only resulted in a slight increase in proliferation at the highest dose of filgrastim. CTV-1 cells showed very mild proliferation, but only after treatment with lenograstim for 72 h at high concentrations (50 and 100 ng/mL). U266 cells showed no proliferation upon treatment with G-CSF (Fig. 2).
The EdU assay confirmed that, when compared to unstimulated controls, lenograstim and filgrastim (both at 10 ng/mL) increased the proliferation of Kasumi-1 cells (from 38.7% to 51.9% and 51.5%, respectively), whereas CTV-1 cells did not respond to treatment. Both filgrastim and lenograstim significantly stimulated the proliferation of AML1/ETO+ Kasumi-1 cells in a time-dependent manner (Fig. 3).
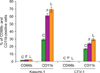
The differentiation effect was determined by analysis of mature granulocyte phenotype marker expression (CD11b and CD66b) by flow cytometry. G-CSF treatment doubled the proportion of CD11b-positive cells in Kasumi-1 cells, whereas the proportion of CD11b-positive cells only mildly increased in CTV-1 cells after 14 d of incubation with G-CSF at 100 ng/mL. Although CD11b-positive cells also increased in unstimulated control cells (29%), the increase in CD11b-positive cells was much more prominent in Kasumi-1 cells after filgrastim and lenograstim treatment (61% and 69%, respectively) (Fig. 4). Expression of CD66b was not significantly affected in either cell line regardless of the concentration of G-CSF, type of G-CSF, or incubation time (Fig. 4).
Many growth factors play pivotal roles in cell proliferation, migration, and differentiation [14]. Although a possible stimulating influence on leukemic cells remains questionable, most studies have reported that G-CSF is a safe agent that improves neutrophil count, thereby reducing the incidence of documented infection without regrowth of leukemic cells or other negative effects [15-18]. In the present study, we evaluated the effects of 2 forms of recombinant human G-CSF (rhG-CSF) available for clinical use: filgrastim is derived from Escherichia coli and has a non-glycosylated form, whereas lenograstim is derived from Chinese hamster ovary (CHO) cells and is glycosylated. The peak serum concentrations of G-CSF after administration of a standard dose of G-CSF (5 µg/kg) is found to range from 15 to 30 ng/mL [19, 20]; therefore, we used a range of G-CSF concentrations spanning this concentration (0, 10, 50, and 100 ng/mL). The proliferation effect of G-CSF was prominent in Kasumi-1 cells, and the 2 forms of G-CSF showed similar effects. This might be due to the high expression of G-CSFR in AML1/ETO+ Kasumi-1 cells, as reported in previous studies [2, 5]. Meanwhile, the proliferation was not stimulated in CTV-1 cells or U266 cells, which did not express G-CSFR. K562 cells with low-level expression of G-CSFR mRNA showed mild proliferation only at 100 ng/mL filgrastim after 72 h; however, this concentration cannot be applied in a clinical setting and is also much higher than the estimated serum concentrations in patients after G-CSF administration.
In addition to a proliferative effect, we noted that G-CSF induced differentiation in AML1/ETO-positive cells with a high level of G-CSFR expression. There have been several reports on the differentiation effect of G-CSF, evidenced by morphologic changes and immunophenotypic changes [21-25]. In the present study, although mild changes in CD11b expression were observed in unstimulated control cells and in stimulated CTV-1 cells, there was a prominent increase of CD11b expression in G-CSF-treated Kasumi-1 cells. However, the expression of CD66b was not affected. During the normal process of differentiation of neutrophils in bone marrow, CD66b is expressed from CD34-myeloblasts, reaching its highest level of expression at the myelocyte stage and decreasing thereafter [26], whereas CD11b is expressed at a later stage and its expression increases during maturation [27]. Thus, the differentiation pattern found in the present study (CD11b+/CD66b-) would not be seen in a normal process and suggests that the in vitro differentiation induced by G-CSF was abnormal and incomplete. Induction of CD11b expression by G-CSF has been previously reported [22, 23]. Given that not all patients with AML1/ETO+ AML show prominent differentiation in response to exogenous G-CSF, it is inferred that other factors such as microenvironments have to be taken into consideration for differentiation in vivo.
Many studies have demonstrated the expression of G-CSFR in tumor cells or autocrine secretion of G-CSF in non-hematopoietic tumors such as colon cancer, ovarian cancer, squamous cell cancer, malignant melanoma, and sarcoma [6-13, 28-30]. In these reports, G-CSF was shown to stimulate proliferation and angiogenesis, and subsequently enhance malignant potential [6, 13]. Owing to the potential risk of stimulation of proliferation by G-CSF, information concerning G-CSFR expression in tumor cells would be helpful in the management of cancer patients. Here, we performed quantitative G-CSFR mRNA expression analyses in various solid tumor cell lines. Among the solid tumors, 13.1% expressed G-CSFR. Of note, G-CSFR expression in the hepatoblastoma cell line HepG2 was high and comparable to that in the AML1/ETO+ Kasumi-1 cell line [2, 5]. However, such expression, especially when low, should be confirmed through additional testing and the statistical relevance of low expression needs to be validated.
In conclusion, G-CSFR expression and the proliferative effects of G-CSF on various malignant cells were demonstrated in the present study. Therefore, G-CSF should be used with caution in patients with hematopoietic or non-hematopoietic tumors with high G-CSFR expression. Accordingly, we suggest that screening for G-CSFR before administering G-CSF would be helpful in minimizing the risk of tumor proliferation. Expression levels of G-CSFR in primary tumor tissues should be evaluated by further study.
Figures and Tables
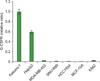 | Fig. 1Expression of G-CSFR in hematologic malignancies and solid tumors using real-time PCR. Among hematologic malignancies, Kasumi-1 and K562 expressed G-CSFR mRNA whereas CTV-1 and U266 did not. Among 38 solid tumor cell lines, 5 cell lines (13.1%) expressed G-CSFR mRNA. The G-CSFR expression of each cell type was normalized to GAPDH expression. The relative expressions were presented as relative ratios compared to gene expression in Kasumi-1 cells (set to 1.0). Results shown are mean values from 3 experiments. |
 | Fig. 2Proliferation effects of lenograstim (left column) and filgrastim (right column) on Kasumi-1, CTV-1, K562, and U266 cells at different concentrations (10, 50, and 100 ng/mL) after 72 h-incubation. The relative proliferation was expressed as percentage of unstimulated control cells (set to 100%). |
Notes
This work was supported in part by (1) Basic Science Research Program through the National Research Foundation of Korea (NRF) Funded by the Ministry of Education, Science and Technology (2012-0002257), (2) a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120216) and (3) a grant (10172KFDA993) from Korea Food & Drug Administration in 2012.
References
1. Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): implications for future therapeutical strategies. Eur J Haematol. 2004. 72:89–106.

2. Moon HW, Shin S, Kim HY, et al. Therapeutic use of granulocyte-colony stimulating factor could conceal residual malignant cells in patients with AML1/ETO+ acute myelogenous leukemia. Leukemia. 2006. 20:1408–1413.

3. Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007. 147:400–411.

4. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991. 78:2791–2808.

5. Shimizu K, Kitabayashi I, Kamada N, et al. AML1-MTG8 leukemic protein induces the expression of granulocyte colony-stimulating factor (G-CSF) receptor through the up-regulation of CCAAT/enhancer binding protein epsilon. Blood. 2000. 96:288–296.

6. Hirbe AC, Uluckan O, Morgan EA, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007. 109:3424–3431.

7. Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994. 56:853–857.

8. Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002. 297:1058–1061.

9. Noda I, Fujieda S, Ohtsubo T, et al. Granulocyte-colony-stimulating factor enhances invasive potential of human head-and-neck-carcinoma cell lines. Int J Cancer. 1999. 80:78–84.

10. Obermueller E, Vosseler S, Fusenig NE, Mueller MM. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004. 64:7801–7812.

11. Savarese TM, Mitchell K, McQuain C, et al. Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett. 2001. 162:105–115.

12. Yang X, Liu F, Xu Z, et al. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J. 2005. 81:333–337.

13. Morales-Arias J, Meyers PA, Bolontrade MF, et al. Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer. 2007. 110:1568–1577.

14. Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 2011. 46:265–273.

15. Ohno R, Tomonaga M, Kobayashi T, et al. Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia. N Engl J Med. 1990. 323:871–877.

16. Heil G, Hoelzer D, Sanz MA, et al. The International Acute Myeloid Leukemia Study Group. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. Blood. 1997. 90:4710–4718.

17. Godwin JE, Kopecky KJ, Head DR, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood. 1998. 91:3607–3615.

18. Usuki K, Urabe A, Masaoka T, et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol. 2002. 116:103–112.

19. Kroger N, Sonnenberg S, Cortes-Dericks L, Freiberger P, Mollnau H, Zander AR. Kinetics of G-CSF and CD34+ cell mobilization after once or twice daily stimulation with rHu granulocyte-stimulating factor (lenograstim) in healthy volunteers: an intraindividual crossover study. Transfusion. 2004. 44:104–110.

20. Hernandez-Bernal F, Garcia-García I, Gonzalez-Delgado CA, et al. Bioequivalence of two recombinant granulocyte colony-stimulating factor formulations in healthy male volunteers. Biopharm Drug Dispos. 2005. 26:151–159.

21. Camera A, Volpicelli M, Villa MR, Risitano AM, Rossi M, Rotoli B. Complete remission induced by high dose erythropoietin and granulocyte colony stimulating factor in acute erythroleukemia (AML-M6 with maturation). Haematologica. 2002. 87:1225–1227.
22. Da Silva N, Meyer-Monard S, Menot ML, et al. Functional G-CSF pathways in t(8;21) leukemic cells allow for differentiation induction and degradation of AML1-ETO. Hematol J. 2000. 1:316–328.

23. Ferrara F, Di Noto R, Viola A, et al. Complete remission in acute myeloid leukaemia with t(8;21) following treatment with G-CSF: flow cytometric analysis of in vivo and in vitro effects on cell maturation. Br J Haematol. 1999. 106:520–523.

24. Nimubona S, Grulois I, Bernard M, et al. Complete remission in hypoplastic acute myeloid leukemia induced by G-CSF without chemotherapy: report on three cases. Leukemia. 2002. 16:1871–1873.

25. Takamatsu Y, Miyamoto T, Iwasaki H, Makino S, Tamura K. Remission induction by granulocyte colony-stimulating factor in hypoplastic acute myelogenous leukemia complicated by infection. A case report and review of the literature. Acta Haematol. 1998. 99:224–230.

26. Hernandez-Campo PM, Almeida J, Matarraz S, de Santiago M, Sanchez ML, Orfao A. Quantitative analysis of the expression of glycosylphosphatidylinositol-anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytometry B Clin Cytom. 2007. 72:34–42.
27. van Lochem EG, van der Velden VH, Wind HK, et al. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B Clin Cytom. 2004. 60:1–13.

28. Ichiishi E, Yoshikawa T, Kogawa T, Yoshida N, Kondo M. Possible paracrine growth of adenocarcinoma of the stomach induced by granulocyte colony stimulating factor produced by squamous cell carcinoma of the oesophagus. Gut. 2000. 46:432–434.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download