Abstract
Interdigitating dendritic cell sarcoma (IDCS) is a very rare and aggressive neoplasm that arises from antigen presenting cells. IDCS usually involves lymph nodes; however, extra-nodal involvement has also been reported. Because a consistent standard therapy for IDCS has not been established to date, we report a case of the successful treatment of disseminated IDCS using ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, and dacarbazine). A 64-year-old man was diagnosed with IDCS on the basis of immunohistochemical findings of a biopsy specimen of the inferior nasal concha. Immunohistochemical staining showed a positive reaction for CD68, leukocyte common antigen, and S-100 protein, but a negative reaction for CD34, CD1a, and CD21. Imaging studies showed cervical and axillary lymphadenopathies, subcutaneous nodules, and a soft tissue lesion in the nasal cavity. Treatment with the ABVD regimen resulted in complete remission after 8 cycles of chemotherapy.
Interdigitating dendritic cell sarcoma (IDCS) is a very rare neoplasm arising from interdigitating reticular cells, which participate in the immune response as antigen presenting cells that stimulate T lymphocytes [1, 2]. Tumor occurrence is usually seen at T-cell rich areas of lymph nodes in the cervical, mediastinal, and axillary regions; however, the involvement of extra-nodal sites such as the spleen, testis, urinary bladder, and pleura have also been reported [3-6]. IDCS is associated with an aggressive clinical course. Although various treatment modalities, including surgery, radiation therapy, chemotherapy, and combinations of these, have been tried, to date there is no consensus on the preferred treatment. Among chemotherapeutic regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), DHAP (dexamethasone, cisplatin, and high-dose cytarabine), EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), ICE (ifosfamide, carboplatin, and etoposide), and cisplatin/epirubicin [1, 2, 7], only ABVD, currently used for the treatment of Hodgkin's lymphoma, has been successful for the treatment of disseminated IDCS [8].
Until now, 2 cases of IDCS have been reported in Korea [3, 9]; 1 patient with localized IDCS showed a nearly complete response to a combination of CHOP chemotherapy and adjuvant radiation therapy [9]; however, the other patient, who presented with extra-nodal involvement of the pleura, died of progressive disease after 2 cycles of CHOP and 1 cycle of IMEP (ifosfamide, methotrexate, etoposide, and prednisolone) [3].
We report the first case in Korea of successful disseminated IDCS treatment using only ABVD chemotherapy.
A 64-year-old man presented to the primary care physician with a 1-year history of nasal congestion. He was referred to our hospital because of abnormal physical findings in the nasal cavity. Other than hypertension, the patient had no medical or surgical history. Rhinoscopic examination showed an ulcerative lesion in the inferior concha, and physical examination indicated multiple lymphadenopathies in both axillae and subcutaneous nodules in the left back. Except for thrombocytosis (472×109/L), laboratory tests did not show abnormal findings. After obtaining written informed consent, an excisional biopsy of the inferior concha was performed. The lesion showed diffuse infiltration of spindle cells, large pleomorphic cells, foamy histiocytes, lymphocytes, and plasma cells (Fig. 1A). The distinction between inflammatory conditions, such as rhinoscleroma, and neoplastic lesions, such as Rosai-Dorfman disease, IDCS, Langerhans cell histiocytosis (LCH), and follicular dendritic cell sarcoma (FDCS), was difficult; therefore, various immunohistochemical studies were performed. On the basis of the immunohistochemical results, i.e., a strong positive reaction for CD68, lysozyme, LCA, and S-100 protein but a negative reaction for CD34, CD1a, smooth muscle actin, and CD21 (Fig. 1B), IDCS was diagnosed.
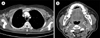
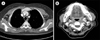
Computed tomography (CT) scans of the chest, abdomen, and pelvis showed multiple enhancing nodules in the subcutaneous layer of the back, with lymphadenopathies in both axillae (Fig. 2A). A head and neck CT scan showed a soft tissue attenuating lesion in the right anterior ethmoid and nasal cavities and multiple lymphadenopathies on both sides of the neck level II (Fig. 2B). Bone marrow involvement was not observed. The lesion in the nasal cavity was partially removed by conchotomy. Further to the previously reported successful treatment of IDCS with ABVD chemotherapy [8], the same ABVD doses (25 mg/m2 adriamycin, 10 mg/m2 bleomycin, 6 mg/m2 vinblastine, and 375 mg/m2 dacarbazine) were infused on days 1 and 15 every 4 weeks. During chemotherapy, no significant complications were observed. After 8 cycles, CT scans of the chest, abdomen, pelvis, and neck showed complete resolution in the lymph nodes of both axillae and the subcutaneous nodules (Fig. 3A). Lesions in the nasal cavity and cervical lymphadenopathies also showed complete resolution (Fig. 3B). CT and PET scans showed no evidence of relapse after 1 year.
Dendritic cell neoplasms are rare tumors, but they are being diagnosed with increasing frequency. The World Health Organization (WHO) classifies dendritic cell neoplasms into 5 groups: LCH, Langerhans cell sarcoma, IDCS, FDCS, and not specified otherwise [10].
Among the tumors arising from reticular cells, IDCS is difficult to diagnose because of histopathologic features that are similar to other tumor types; therefore, a high index of suspicion is required, particularly in cases with extra-nodal involvement, considering its rarity. Histological findings of an excisional biopsy specimen from the nasal concha showed diffuse infiltration of spindle-shaped cells and pleomorphic cells, in addition to foamy cells with various inflammatory cells. Tumor cells in LCH are positive for CD1a and S-100 protein, whereas tumor cells in FDCS are positive for CD21, CD35, and clusterin. In this case, the tumor cells were positive for CD68, lysozyme, LCA, and S-100 protein but were negative for CD21 and CD1a; these findings were consistent with the IDCS immunophenotype [1, 2, 11-14].
Wide dissemination of tumors, as in this patient, has been described in most cases of IDCS. The age range in reported cases of IDCS is 61-88 years with a mean age of 71.2 years [1].
Because of its rarity, data regarding the clinical behavior of IDCS and treatment outcomes are relatively scarce. In patients with localized disease, surgical resection has been reported to be the mainstay of treatment; however, a recurrence rate of up to 40% has been reported [10]. The role of chemotherapy and radiotherapy in IDCS treatment has not been established. To date, chemotherapy for IDCS includes the use of chemotherapeutic regimens for non-Hodgkin's disease or Hodgkin's disease; however, results of these combination chemotherapies have been very disappointing. The duration of remission was usually short with frequent recurrences. Because of a lack of clinical data, high-dose chemotherapy and autologous bone marrow transplantation have also not been recommended [1, 2, 11, 15].
The ABVD regimen is the only treatment known to have resulted in complete remission for disseminated IDCS to date [8]. Here, we report the first case in Korea of the successful treatment of disseminated IDCS with 8 cycles of chemotherapy using the ABVD regimen. Further studies are warranted to arrive at a consensus for the successful treatment of IDCS. However, the case reported here highlighted the efficacy of the ABVD regimen, and we recommend ABVD chemotherapy as a feasible treatment option for disseminated IDCS in the future.
Figures and Tables
 | Fig. 1Histological features of the nasal cavity include diffuse infiltration of spindle cells, large pleomorphic cells, foamy histiocytes, and various inflammatory cells (A). Immunohistochemical staining shows a strong positive reaction for S-100 protein (B). |
References
1. Fonseca R, Yamakawa M, Nakamura S, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol. 1998. 59:161–167.

2. Kairouz S, Hashash J, Kabbara W, McHayleh W, Tabbara IA. Dendritic cell neoplasms: an overview. Am J Hematol. 2007. 82:924–928.

3. Han HS, Lee OJ, Lim SN, et al. Extranodal interdigitating dendritic cell sarcoma presenting in the pleura: a case report. J Korean Med Sci. 2011. 26:304–307.

4. Kawachi K, Nakatani Y, Inayama Y, Kawano N, Toda N, Misugi K. Interdigitating dendritic cell sarcoma of the spleen: report of a case with a review of the literature. Am J Surg Pathol. 2002. 26:530–537.
5. Luk IS, Shek TW, Tang VW, Ng WF. Interdigitating dendritic cell tumor of the testis: a novel testicular spindle cell neoplasm. Am J Surg Pathol. 1999. 23:1141–1148.
6. Rupar G, Beham-Schmid C, Gallé G, Zigeuner R, Langner C. Interdigitating dendritic cell sarcoma of urinary bladder mimicking large intravesical calculus. Urology. 2005. 66:1109.

7. Gaertner EM, Tsokos M, Derringer GA, Neuhauser TS, Arciero C, Andriko JA. Interdigitating dendritic cell sarcoma. A report of four cases and review of the literature. Am J Clin Pathol. 2001. 115:589–597.
8. Olnes MJ, Nicol T, Duncan M, Bohlman M, Erlich R. Interdigitating dendritic cell sarcoma: a rare malignancy responsive to ABVD chemotherapy. Leuk Lymphoma. 2002. 43:817–821.

9. Kim SY, Kang JH, Chun SH, et al. Interdigitating dendritic cell sarcoma of the tonsil. Asia Pac J Clin Oncol. 2010. 6:144–148.

10. De Pas T, Spitaleri G, Pruneri G, et al. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008. 65:1–7.

11. Rousselet MC, François S, Croué A, Maigre M, Saint-André JP, Ifrah N. A lymph node interdigitating reticulum cell sarcoma. Arch Pathol Lab Med. 1994. 118:183–188.
12. Weiss LM, Beckstead JH, Warnke RA, Wood GS. Leu-6-expressing cells in lymph nodes: dendritic cells phenotypically similar to interdigitating cells. Hum Pathol. 1986. 17:179–184.

13. Weiss LM, Berry GJ, Dorfman RF, et al. Spindle cell neoplasms of lymph nodes of probable reticulum cell lineage. True reticulum cell sarcoma? Am J Surg Pathol. 1990. 14:405–414.

14. Wood GS, Turner RR, Shiurba RA, Eng L, Warnke RA. Human dendritic cells and macrophages. In situ immunophenotypic definition of subsets that exhibit specific morphologic and microenvironmental characteristics. Am J Pathol. 1985. 119:73–82.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download