Abstract
Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is a distinctive lymphoma characterized by an infiltration of subcutaneous tissue by neoplastic cytotoxic T cells. There was no distinction between TCR alpha/beta phenotype and TCR gamma/delta phenotype, and anthracycline-based chemotherapy was usually used for both. Here, we report a patient with recurrent SPTL who achieved a second long-term complete remission by repeated cyclosporine A (CsA) treatment. From 2000 to 2001, the patient received anthracycline-based combination chemotherapy. However, the treatment did not induce long-term remission. In 2002, he received cyclosporine treatment for about 6 months. This resulted in a 5-year remission that ended in relapse in 2008. He received CsA treatment once again and attained a second long-term remission. This case suggests that re-treatment with CsA can be a good option for relapsed SPTL cases and can result in long-term remission.
Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is a distinctive skin lymphoma similar to panniculitis that is characterized by an infiltration of subcutaneous tissue by neoplastic cytotoxic T cells [1]. It mainly involves the extremities and trunk, and it presents with subcutaneous masses or flat plaques [2]. The patients often exhibit B-symptoms (fever and weight loss), severe fatigue, pancytopenia, and disseminated intravascular coagulation [3].
Recently, SPTL has been classified as 2 subtypes: T-cell receptor (TCR) alpha/beta phenotype and TCR gamma/delta phenotype. According to the recent World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) classification, SPTL is restricted to cases in which the TCR alpha/beta phenotype is expressed.
SPTL expressing the alpha/beta phenotype shows subcutaneous infiltrates that spare the epidermis and dermis. Treatment outcomes are excellent and show a 5-yr overall survival rate of 82% [3]. By contrast, primary cutaneous gamma/delta T-cell lymphoma expressing the gamma/delta phenotype is a disseminated disease that presents with frequent mucosal and extranodal involvement. This case involved the dermis, epidermis, and fat, with rimming of fat globules and angioinvasion. The patient also developed hemophagocytic syndrome. Most of patients with hemophagocytic syndrome had poor outcomes despite aggressive chemotherapy; the median survival was 15 mo [4].
This case of SPTL was diagnosed when the detailed criteria that subdivide SPTL into SPTL and primary cutaneous gamma/delta T-cell lymphoma were not available. At that time, SPTL was treated with doxorubicin-based chemotherapy. However, this case relapsed less than 1 yr after the chemotherapy. This patient was treated with cyclosporine A (CsA) as a second salvage chemotherapy and reached a 5-yr long remission. Unlike SPTL, which has favorable clinical features, this case was accompanied by angioinvasion and hemophagocytic syndrome. According to the new guidelines, this case is immunohistochemically consistent with the alpha/beta phenotype SPTL, but has some of the clinical features of the gamma/delta phenotype.
Five years after remission, the patient relapsed again with skin nodules over the whole body. Treatment with repeated CsA reached CR. There is no evidence of disease 3 yr after retreatment with CsA.
This report describes a case of subcutaneous panniculitis-like T-cell lymphoma with clinically poor prognostic factors that was successfully treated with CsA.
In August of 2000, a 18-yr-old male was admitted with a 1-mo history of fever and multiple subcutaneous nodules on the trunk and extremities. There were no other notable findings on physical examination. Imaging studies including chest X-ray, computed tomography (CT) scan of abdomen, and brain magnetic resonance imaging (MRI) scans were performed for fever of unknown origin (FUO), and there was no involvement of other organs except splenomegaly. Complete blood count (CBC) findings were consistent with mild anemia and leukopenia (Hb level, 10.2 g/dL; leukocyte count, 1.28×109/L; and platelet count, 164×109/L). Biochemical tests revealed elevated levels of aspartate aminotransferase (AST; 73 IU/L), alanine aminotransferase (ALT; 59 IU/L), and lactate dehydrogenase (LDH; 752 IU/L), and ferritin >1,000 µg/L.
Immunohistochemical staining was performed on a biopsy specimen from a skin nodule on the leg, which showed atypical lymphocyte infiltrations in a lobular panniculitis pattern (Fig. 1A). The atypical lymphocytes were positive for CD3 (Fig. 1C) and CD45RO and negative for CD20 and CD56. There was fat rimming (Fig. 1B) with CD8-positive atypical lymphocytes (Fig. 1D). An analysis of TCR gamma gene rearrangement using the skin specimen was negative. This diagnosis was SPTL. Bone marrow aspiration showed hemophagocytic histiocytes. There was no evidence of bone marrow involvement of the lymphoma.
This patient was treated with a COPBLAM-V (cyclophosphamide/vincristine/prednisolone/bleomycin/doxorubicin) regimen. After 1 cycle of chemotherapy, fever, leukopenia, and nodules disappeared. After 4 cycles of the chemotherapy, the patient declined further chemotherapy, and did not return to the clinic. One year later, he returned with relapsed fever and skin rash with nodules. He showed pancytopenia (Hb level, 9.7 g/dL; leukocyte count, 0.72×109/L; and platelet count, 84×109/L). He was treated with modified CHOP (cyclophosphamide/doxorubicin/vincristine/prednisolone) regimen for 4 mo. The skin nodules persisted. Re-biopsy of the skin indicated that SPTL remained. The disease was thought to be refractory to chemotherapy.
Treatment with CsA 200 mg/d was attempted, and the patient achieved remission after 1 mo. The treatment was maintained for 12 wk, and then the dose was slowly tapered for 6 wk. As a result, a complete remission (CR) was achieved. For the next 4 yr, no recurrences were observed. Five years after the achievement of CR, the patient presented with palpable masses in posterior neck, chest wall, back, and abdomen. Whole body PET-CT scan and CT scan of the abdomen revealed multiple metastatic lesions (Fig. 2A). Laboratory findings showed leukopenia (3.1×109/L), abnormal liver function (AST/ALT (IU/L): 75/102), and elevated LDH (436 IU/L). After CsA 400 mg/d for 4 d during a 1-mo period, no response was noted. Therefore, cisplatin/cytarabine combination chemotherapy was administered up to 2 cycles, but the skin lesions remained. Finally, he was treated with CsA 800 mg/d for 7 d. When he returned to the clinic, the lesions markedly improved. PET-CT scan showed no definite evidence of disease (Fig. 2B). We considered the disease status as CR, and continued CsA at 400 mg/d over 10 wk. There has been no evidence of recurrence 3 yr after retreatment.
SPTL usually presents with multiple erythematous subcutaneous nodules, and the natural course of SPTL is highly variable. Hemophagocytic syndrome [5], low white blood cell count, or elevated LDH [6] have been known to be poor prognostic indicators. We diagnosed this patient as having SPTL and excluded primary cutaneous gamma-delta T-cell lymphoma based on immunohistochemistry results (positive staining for CD8 and negative staining for CD56) and negative result of TCR gamma rearrangement analysis [3]. This case also met the hemophagocytic lymphohistiocytosis (HLH) diagnostic criteria [7] (fever, splenomegaly, hemophagocytosis in bone marrow, ferritin ≥500 µg/L, and cytopenia). The cytopenia criteria were not met at diagnosis, but were met at relapse in 2001. We demonstrated that a case of SPTL with clinical features indicating poor prognosis could be treated with CsA resulting in long-term remission.
These findings suggested that CsA can be a good treatment choice in SPTL that is refractory to cytotoxic chemotherapy. A wide variety of treatment modalities for SPTL have been reported, ranging from corticosteroids and immunosuppressive agents to radiotherapy and combination chemotherapy [2, 3, 5]. The anthracycline-based regimen is commonly used, and produces long-term remission in a subset of patients [5]. Therefore, at the time of diagnosis 10 yrs ago, anthracycline-based regimens like CHOP therapy were routinely chosen. In this case, it is hard to determine the effects of the initial anthracycline-based chemotherapy on the disease because the patient did not complete the entire planned chemotherapy course. However, the second line cytotoxic chemotherapy after relapse did not induce a complete response, which suggests that CsA treatment could be much more effective for this disease than cytotoxic chemotherapy. Our results are similar to another report [3] that describes the use of a second-line cytotoxic agent for SPTL that failed produce a good result.
Recently, there have been other reports that CsA therapy might be a good option for the treatment of SPTL relapsed after chemotherapy [8]. In these reports, 3 out of 4 cases that recurred following CHOP, ESHAP, or fludarabine-based regimen chemotherapy, achieved CR with CsA therapy; 1 patient died of severe infection.
Molecular studies have revealed monoclonal T-cell receptor gene rearrangement in 85% of reported SPTLs [5], but in this case, monoclonal T-cell receptor gene rearrangement was not detected. Similarly, in all 3 previous cases showing response to CsA therapy after relapse, T-cell receptor gene rearrangement was either negative or not evaluated [8].
This finding suggests a possibility that SPTL has an independent disease entity that is distinct from other types of T-cell lymphoma. To our knowledge, this is the first case of SPTL with long-term follow-up after CsA treatment. At present, further large studies are warranted to validate the effects of CsA on SPTL as a first or second line therapy.
In summary, we report a case of subcutaneous panniculitis-like T-cell lymphoma that relapsed twice with poor prognostic factors, and where CsA retreatment resulted in long-term remission. This case suggests that CsA can be a good treatment option for select cases of SPTL.
Figures and Tables
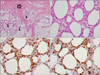 | Fig. 1Biopsy of a skin nodule. (A) The skin tissue showed dense lymphocytic infiltrate in lobular panniculitis-like pattern (arrowheads) with focal dermal infiltrations (arrow) (hematoxylin and eosin stain, ×40 magnification). (B) The infiltrated lymphocytes showed atypical features with hyperchromatic, irregular nuclei, and occasional nucleoli. There was fat rimming with atypical lymphocytes (hematoxylin and eosin stain, ×400 magnification). (C) In immunohistochemical stain, the atypical lymphocytes were positive for CD3 (CD3, ×400) and (D) CD8 (CD8, ×400 magnification). |
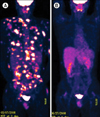 | Fig. 2Whole body PET-CT (A) At the second relapse, multiple nodules with high signal intensities were noted in multiple subcutaneous and muscular areas. (B) After treatment with CsA, no active lesions were seen in the PET-CT scan. Abbreviations: PET-CT, positron emission tomography-computed tomography; CsA, cyclosporine A. |
References
1. Gonzalez CL, Medeiros LJ, Braziel RM, Jaffe ES. T-cell lymphoma involving subcutaneous tissue. A clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991. 15:17–27.
2. Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009. 133:303–308.

3. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008. 111:838–845.

4. Foss FM, Edelson RL, Wilson LD. DeVita VT, Lawrence TS, Rosenberg SA, editors. Lymphomas: Cutaneous T-cell lymphomas. DeVita, Hellman, and Rosenberg's cancer: Principle & practice of oncology. 2005. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2143–2158.
5. Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004. 101:1404–1413.

6. Huang JJ, Cai MY, Ye S, Li ZM, Huang HQ, Lin TY. Clinical analysis of 19 cases of subcutaneous panniculitis T-cell lymphoma with literature review. Ai Zheng. 2009. 28:1093–1099.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download