Abstract
Background
Arsenic trioxide (As2O3) is a well-known and effective treatment that can result in clinical remission for patients diagnosed with acute promyelocytic leukemia (APL). The biologic efficacy of As2O3 in APL and solid tumor cells has been explained through its actions on anti-proliferation, anti-angiogenesis, and apoptotic signaling pathways. We theorize that As2O3 activates a pathway that disrupts microtubule dynamics forming abnormal, nonfunctioning mitotic spindles, thus preventing cellular division. In this study, we investigated how As2O3 induces apoptosis by causing microtubule dysfunction.
Methods
Cultured NB4 cells were treated with As2O3, paclitaxel, and vincristine. Flow cytometric analysis was then performed. An MTT assay was used to determine drug-mediated cytotoxicity. For tubulin polymerization assay, each polymerized or soluble tubulin was measured. Microtubule assembly-disassembly was measured using a tubulin polymerization kit. Cellular microtubules were also observed with fluorescence microscopy.
Results
As2O3 treatment disrupted tubulin assembly resulting in dysfunctional microtubules that cause death in APL cells. As2O3 markedly enhanced the amount of depolymerized microtubules. The number of microtubule posttranslational modifications on an individual tubulin decreased with As2O3 concentration. Immunocytochemistry revealed changes in the cellular microtubule network and formation of polymerized microtubules in As2O3-treated cells.
Arsenic compounds were used therapeutically by the ancients and are also widely utilized in traditional Chinese medicine. Arsenic trioxide (As2O3) is a cancer therapeutic that can be used alone or in combination with other agents to treat patients with acute promyelocytic leukemia (APL). Notably, As2O3 treatment has been known to achieve high rates of remission. In newly diagnosed patients, complete remission rates have ranged from 86% to 95%, and, in relapsed patients, rates of 80% to 93% have been reported [1]. Moreover, As2O3 has shown to exhibit anticancer properties in diseases such as multiple myeloma and myelodysplastic syndromes. As such, there has been great interest in As2O3 as a chemotherapeutic drug. Arsenic affects numerous intracellular signal transduction pathways and causes many alterations in cellular function. These actions of arsenic may result in the induction of apoptosis, the inhibition of growth and angiogenesis, and the promotion of differentiation [2]. However, the mechanism whereby arsenic specifically targets tumor cells is not clearly understood.
Of the various target elements affected by arsenic, microtubules are critical elements in a wide variety of fundamental cell functions, including mitosis, motility, and intracellular vesicular transport [3]. As such, microtubules are a good target for many natural toxins as well as chemotherapeutic drugs [4, 5]. Disruption of microtubule dynamics induces abnormal formation or loss of a functional mitotic spindle, both of which interrupt cell division and cause cell death. Several lines of evidence have previously been reported to suggest that As2O3 affects spindle formation/function by modulating assembly and/or disassembly of tubulins in several cancer cell lines, including leukemic cells [6, 7]. Together, these data suggest that As2O3 may mimic microtubule poisons and interrupt mitosis [7].
To date, a few reports have indicated that, depending on the cell line, As2O3 treatment results in cell-cycle arrest at the G1 or G2/M phase via microtubule dysfunction [8]. Other contrastive opinions for the As2O3 mechanism of action on the assembly/disassembly of tubulin in tumor cell lines have also been provided [7-9]. Li and Broome reported that As2O3 treatment resulted in cell-cycle arrest at metaphase in myeloid leukemia cells through noncompetitive disruption of GTP binding to β-tubulin and inhibition of GTP-induced tubulin polymerization [9]. Ling et al. reported that As2O3 caused a paclitaxel-like, concentration-dependent, tubulin polymerization in vitro and altered the microtubule network as determined by cell morphology and electron microscopy (EM) analysis [8].
In this study, we wanted to show that As2O3 might induce apoptosis by causing microtubule dysfunction. We investigated whether As2O3 promotes or inhibits the polymerization of microtubules in an APL cell line. Thus, we hoped to better understand the unique mechanism underlying the action of As2O3.
Acute promyelocytic leukemia (APL) NB4 cells (provided by Dr. J. H. Won of Soon Chun Hyang University College of Medicine, Korea) were maintained as suspension cultures in PRMI-1640 (Gibco, Invitrogen Ltd, UK) containing 10% fetal bovine serum (Gibco, Invitrogen Ltd, UK) and 1× penicillin-streptomycin (Gibco, Invitrogen Ltd, UK). Cells were maintained at 37℃ in water-saturated atmosphere containing 5% CO2. As2O3 was purchased from Sigma Chemical Inc. and dissolved in 1 M NaOH as stock solution. Paclitaxel was purchased from BMS Pharmaceutical Korea Limited and a stock solution of paclitaxel (Taxol®, Korea) was prepared as per the manufacturer's suggestion. Vincristine was purchased from Reyon Pharmaceutical Co., LTD and prepared at a concentration of 0.1 M in PBS. Monoclonal anti-α-tubulin, anti-acetylated α-tubulin, anti-glycealdehyde-3-phosphate dehydrogenase (GAPDH), and HRP-conjugated secondary antibody were purchased from Santa Cruz Biotechnology, Inc., CA, USA.
NB4 cells (2×106 cells) were treated with each drug at the indicated concentration for 24 hours. Cells were washed with PBS by spinning at 1,000 rpm for 5 min at 4℃ and fixed with 1 mL of ice-cold 95% ethanol drop-wise while vortexing. The fixed cells were incubated in ice-cold 95% ethanol for at least 30 min and then centrifuged as described above. The pellets were resuspended in 1 mL PBS and 100 µL of 1 mg/mL propidium iodide (PI) for 5-10 min at room temperature. Flow cytometric analysis was performed using a FACSCalibur Flow Cytometer and Cell Quest software (BD biosciences, San Jose, CA, USA).
To determine drug-mediated cytotoxicity, an MTT assay was performed. This was carried out using the MTS reagent of the Cell Titer 96 Aqueous One Solution (Promega Corp., USA) with absorbance measured at 490 nm, as previously described [10]. Briefly, NB4 cells were seeded in 0.5×105 cells per well for 24 hrs at 37℃ and 5% CO2. After treatment with the indicated drug concentrations, cells were reincubated for 24 hrs and 48 hrs. Twenty microliters of the MTS solution was added to each well and incubated at 37℃. Thereafter, the absorbance (A) values of each well was determined using a microplate spectrophotometer (Bio-Tek Instruments Inc., USA). The percentage of viable cells was calculated using the background-corrected absorbance as follows:
% cytotoxicity=(1-A of experimental well/A of positive control well)×100
Cells were plated in 24-well plates, grown for 1 day (60-80% confluency), and treated with either no drug or varying concentrations of drugs at 37℃ for 6 hours. After the medium was removed, cells were rinsed in 1× PBS at 22℃, harvested at the same temperature in lysis buffer containing 0.1 M PIPES, 1 mM EGTA, 1 mM MgSO4, 30% glycerol, 5% DMSO, 5 mM GTP, 0.125% NP-40, and protease inhibitor cocktail (Calbiochem, Merck KGaA, Darmstadt, Germany), and then centrifuged at 13,200 rpm at 22℃ for 30 min followed by vortexing, to separate polymerized (P) from soluble (S) tubulin [11]. Pellets of polymerized "P" tubulin were resuspended in a volume of lysis buffer equal to the soluble "S" fraction. Each had gel sample buffer added and equal aliquots separated on 10% SDS-PAGE gels were transferred to PVDF membranes (Whatman). The membranes were blocked with 5% skim milk for 2 hrs in TBS buffer containing 0.1% Tween 20 and either the α-tubulin antibody (SantaCruze Biotechnology, CA, USA) or acetylated α-tubulin (SantaCruze Biotechnology, CA, USA). Antibodies were incubated overnight at 4℃ with constant shaking. After washing, an HRP-conjugated anti-mouse antibody was applied at a dilution of 1:10,000. Secondary antibody binding was carried out at room temperature for 2 hrs with shaking. To visualize the protein bands, chemiluminescence detection was performed with the Chemiluminescent kit (Bio-Rad Laboratories, CA, USA). The blots were reprobed with a monoclonal antibody against GAPDH, and the results were used as loading controls. Band intensities were quantified by densitometry using Bio-Rad software.
The effects of As2O3 on the microtubule assembly-disassembly process were determined using a tubulin polymerization kit (Cytoskeleton, Denver, CO, USA). Purified bovine brain tubulin was resuspended on ice in ice-cold buffer (80 mM PIPES at pH 6.9, 2 mM MgCl2, 0.5 mM EGTA, 15% glycerol, 1 mM GTP) and 100 µL of the suspension (3 mg/mL) was aliquoted into half of a 96-well plate pre-warmed to 37℃ as per the manufacturer's instructions. The assay was determined by measuring the change of absorbance (340 nm) at room temperature using a spectrophotometer every 5 min for 70 min (µQuant, Bio-Tek Instruments Inc., USA).
After treatment with As2O3, paclitaxel, and vincristine for 6 hours, cells were washed with PBS solution and the cell suspension was then incubated on a glass slides coated with poly-L-lysine for 30 min at room temperature. After aspirating the excess cell suspension, the cells were fixed with 3.7% formaldehyde in PBS for 10 minutes and permeabilized with 50 µL of BD Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA, USA) for 25 min at 4℃. Permeabilize, fixed cells were washed twice with 100 µL of 1× BD Perm/Wash buffer (BD Biosciences, San Jose, CA, USA) for 5 min. Fixed cells were incubated with a monoclonal anti-α tubulin antibody (1:50; Santa Cruz Biotechnology, CA, USA) at room temperature for 1 hr. After washing 3 times in 100 µL with 1× BD Perm/Wash buffer (BD Biosciences, San Jose, CA, USA) for 5 min, cells were reincubated with FITC-conjugated second antibody (1:100, Santa Cruz Biotechnology, CA, USA) in the dark for 1 hr. Cellular microtubules were imaged using an Olympus IX-81 fluorescence microscope.
In a previously reported study, As2O3-induced anti-proliferation in various tumor cell lines was evaluated and a clinical study revealed that the effective dose of As2O3 in vivo/vitro was 0.5-3 µM [9, 12]. However, to confirm As2O3 cytotoxicity in NB4 cells, we examined different concentration of As2O3 and measured cytotoxicity via an MTT assay after 24 and 48 hrs of treatment. As shown in Fig. 1A, the cytotoxic effect of As2O3 was dose and time dependent. At 5 µM As2O3, NB4 cell growth was inhibited by more than 50%. We next examined whether the inhibition of cell growth by As2O3 could cause apoptosis. After NB4 cells were treated with the indicated concentration of As2O3 for 24 hrs, As2O3-induced apoptosis was determined by propidium iodide (PI) staining and compared with controls for 5 nM and 7 nM paclitaxel treatments. As shown in Fig. 1B and Table 1, paclitaxel, a microtubule-targeting drug, induced G2/M phase arrest and caused apoptosis. At a low concentration (<1 µM As2O3), <13% of cells were in the G2/M phase but at a concentration of >3 µM, >40% of cells were in the G2/M phase. Similar to paclitaxel treatment, As2O3 treatment resulted in a concentration-dependent accumulation of NB4 cells in the G2/M phase, although As2O3-treated cells resulted in a higher concentration in this phase than when cells were treated with paclitaxel. Cell cycle arrest induced by As2O3 treatment is initiated by the dissipation of the mitochondrial membrane potential (MMP), which leads to the release of cytochrome c (data not shown). Together, these data indicate that As2O3-induced apoptosis was preceded by arrest in the G2/M phase of the cell cycle similar to paclitaxel-induced apoptosis.
Because As2O3 treatment markedly blocked the cell cycle in the G2/M phase, we became interested in testing whether As2O3 would disrupt or enhance tubulin polymerization in NB4 cells. To test this hypothesis, we examined the affects of tubulin polymerization and depolymerization on the treatment at various concentrations of As2O3 for a 6 hours treatment. Vincristine and paclitaxel were used as positive controls and all experiments were performed by separating polymerized "P" from soluble "S" tubulin at 22℃. As shown in Fig. 2A, vincristine or paclitaxel enhanced the cellular levels of the "S" fraction and "P" fraction in the cell lysates, similar to previously published reports. When cells were treated with increasing concentrations of As2O3, the amount of tubulin in the depolymerized fraction, "S", was increased compared with untreated cells. The baseline proportion of α-tubulin in the soluble fraction was 59.6%, while the depolymerized proportion observed after treatment was approximately 52-72%. The tubulin shift to the "S" fraction was exactly noted over the 5-20 µM As2O3 range. As such, the increase in the number of cells in arrest with As2O3 found in the apoptosis study correlated with the increased "S" fraction of tubulin and was greater than that in the control cells. These data clearly indicate that As2O3 induces tubulin depolymerization, although the effect is not as strong as vincristine-induced depolymerization.
Since As2O3 reduced the cytotoxicity of antimicrotubule drugs in SK-N-SH cells [13], we investigated whether the combination of paclitaxel and As2O3 synergistically affected tubulin assembly in NB4 cells. To examine to the synergistic mechanism of As2O3 and paclitaxel treatment on microtubule formation, we treated cells with 5 µM As2O3 alone, 10 µM As2O3 alone, and 40 nM paclitaxel alone, or in combination at the same concentrations for 7 hours. The results shown in Fig. 2B reveal that the combined effects of As2O3 and paclitaxel did not influence the tubulin polymerization and microtubule assembly.
It is well established that tubulin can be altered by post-translational modifications, and several of these are exclusive to α-tubulin. Although the precise relationship between these tubulin modifications and microtubule stability has not been elucidated, several changes are definitive markers of microtubule stability [14, 15]. Therefore, we examined the extent of acetylation of α-tubulin, a posttranslational modification indicative of stabilized microtubules, often observed following exposure of cells to microtubule stabilizing agents (i.e., paclitaxel and epothilone) [16]. As shown in Fig. 3, we assessed the expression levels of modified α-tubulin forms in separated fractions of NB4 cell lysates. The proportions below the bands represent the expression quantity in each fraction. We observed a marked increase in the amount of acetylated α-tubulin (<90%) in the polymerized fraction (P) of paclitaxel treated cells, compared to the controls. In addition, the proportion of polymerized fraction (P) with As2O3 treatment was decreased (<62%) compared to untreated cells. Therefore, these data indicate that As2O3 increases microtubule dynamics by interfering with post-translational modifications involved in microtubule stability.
Because As2O3 treatment markedly increased the disassembled portion of tubulin and influenced the stability of microtubules, we became interested in testing whether As2O3 could directly affect tubulin. To test this hypothesis, tubulin polymerization and depolymerization in vitro were examined at 37℃ in a reaction mixture containing purified tubulin and GTP, in the presence or absence of As2O3. Fig. 4 shows that As2O3 significantly promotes tubulin depolymerization, an effect similar to that induced by vincristine. Thus, these data suggest that As2O3 has directly bounds tubulin and induces tubulin depolymerization.
Because vincristine-like, tubulin-destabilizing activity was found in vitro, we tested whether As2O3 treatment affected the cellular microtubule network. NB4 cells were treated with the indicated concentration of paclitaxel, vincristine, and As2O3 or with no drug-treatment as a control. After 6 hours of incubation, the microtubule network was visualized by immunocytochemistry. As shown in Fig. 5, the microtubule network in control cells exhibited normal cytoskeletal arrangement and organization. Treatment with vincristine resulted in microtubule depolymerization with a decrease in the density of cellular microtubule and disappearance of microtubule bundles surrounding in the cytoplasm. As2O3 treatment resulted in findings similar to those seen with vincristine-induced microtubule changes (e.g., decreased microtubule density). In contrast, paclitaxel treatment induced microtubule polymerization with long, thick cytoplasmic microtubules.
The microtubules are well recognized as a chemotherapeutic target, and there is an ongoing search for high efficacy microtubule targeting agents. In cultured, malignant cells, microtubules contribute to various mechanisms, including drug efflux transporter [17], altered tubulin isotype expression [5, 18], and tubulin mutation [19]. Therefore, it is suggested that arsenic compounds might be useful for the treatment of some instances of human cancer [20].
Our data demonstrates that As2O3 efficiently inhibits proliferation of NB4 cells and, in a previous study, the IC50 value for As2O3 in MCF-7 breast carcinoma and A2780 ovarian carcinoma cell lines was approximately 3 µM, a concentration very close to the effective serum concentration for successfully treating patients with acute promyelocytic leukemia in the clinic [8]. Although it was a different type of cancer cells line, the cytotoxicity of As2O3 at about 50% was in the range of the 5 µM for NB4 cells (Fig. 1A). This evidence indicates that the efficiency of As2O3 was similar to previously described reports. Some investigators also report that As2O3 causes DNA damage, oxidative stress, and mitochondrial dysfunction [21-23]. In addition, As2O3 treatment blocked the cell cycle in these cell line. In the present study, we found that As2O3 treatment resulted in cell-cycle arrest at the G2/M phase in NB4 cells (Fig. 1B).
We also considered whether As2O3-induced G2/M phase arrest is due to its interaction with tubulin, thus resulting in abnormal tubulin polymerization. To test this, we have taken advantage of an assay designed by Minotti et al. [24] to separate polymerized "P" from "S" soluble tubulin fractions by centrifugation. This procedure, and subsequent modifications of it, has been widely utilized to quickly determine the percentage of tubulin polymers in cells under a variety of experimental conditions. An increase in the "S" fraction serves as an indicator of tubulin destabilization. In the present study, we were able to show that the presence of unstable microtubules in the treated cell populations by several criteria. First, compared to the controls, an increased proportion of tubulin was found in the soluble tubulin fractions. Soluble tubulin amounts were present in an As2O3 concentration-dependent manner. The effect of As2O3 on tubulin depolymerization was weaker than that of vincristine (Fig. 2). These data suggest that As2O3 directly or indirectly induces the disruption of microtubule formation via tubulin polymerization in contrast to data published by Ling et al., who reported that As2O3 increases tubulin polymerization [8]. In addition, the disassembly of the microtubule network after treatment with As2O3 suggests that it acts as a microtubule depolymerizing agent. We were able to demonstrate that α-tubulin posttranslational modifications correspond to increased microtubule stability. Tubulin can undergo numerous posttranslational modifications, including phosphorylation, polyglutamylation, polyglyclation, deglutamylation, acetylation, and tyrosination/detyrosination. Although the functions of these α-tubulin modifications remain unclear, deglutamylation, acetylation, and detyrosination are indicative of stable microtubules [14, 15, 25]. Acetylation occurs on lysine 40 near the amino terminus of α-tubulin and does not appear to increase the levels of stable microtubules but instead accumulates in existing stable microtubules [14, 15]. Glu-tubulin is formed when the last residue on α-tubulin, a tyrosine, is removed by tubulin-carboxypeptidase and the glutamic acid is exposed [25]. As such, the phenomenon observed here, depolymerization of microtubule by As2O3, could be explained, in part, by alteration of microtubule stability through microtubule modification. As compared to paclitaxel treatment, As2O3 significantly decreased acetylated α-tubulin (Fig. 3), this suggests that microtubule stabilization may play an important role in As2O3-mediated microtubule depolymerization.
Next, we tested the effect of As2O3, paclitaxel, and vincristine on tubulin assembly in a cell-free in vitro system (Fig. 4). We found that the effect of As2O3 was similar to that of vincristine but different from paclitaxel. This does not rule out the possibility that As2O3 may bind to polymerized tubulins and stabilize microtubules. The reason for the appearance of tubulin depolymerization may be explained if As2O3 acts as a noncompetitive inhibitor, thus interfering with the GTP binding domain on β-tubulin [9]. In this study, it is notable that the same concentration of As2O3 was capable of inhibiting the assembly of tubulin, but these concentrations were also found to elicit some differences in the cell-based assays or the cell-free systems. The intracellular conditions may provide an ideal environment for the reaction of As2O3 with microtubules whereas the reaction conditions of these widely used cell-free microtubule assembly-disassembly assays may not facilitate the As2O3-microtubule interaction. Indeed, this phenomenon has been observed before by using other tubulin ligands, although the discrepancies were not as pronounced as in this study [26].
Finally, indirect immunofluorescence technique allowed us to detect morphological changes in the microtubule network, such as alterations in microtubule organization and arrangement. The changes in microtubule length and density constitute an appropriate method to qualitatively assess the intracellular microtubule polymerization or depolymerization caused by anti-tubulin agents. The results from our tubulin depolymerization experiments indicated that, similar to the effect of vincristine treatment, As2O3 treatment prevented tubulin polymerization. To validate these results, changes in the cellular microtubule network were observing immunocytochemically. We found that the effect of As2O3 was similar to that of vincristine (Fig. 5). Thick bundles of microtubule network surrounding cytoplasm were observed in paclitaxel-treated cells, whereas shortened depolymerized microtubules were observed in As2O3- and vincristine-treated cells.
In summary, our data demonstrate that As2O3 is efficacious in suppressing cell growth in NB4 cells. As2O3 directly or indirectly interferes with microtubules and blocks the cell cycle at the G2/M phase. The effects of As2O3 on microtubule assembly are vincristine-like, but also distinct from vincristine. As2O3 is less active than vincristine in NB4 cells and is diametrically opposite of the observations published by Ling et al. who reported that As2O3 induced tubulin polymerization [8]. The unique mechanism that allows for As2O3 to induce both cell death and cell cycle arrest, and to cause microtubule depolymerization, makes it an ideal candidate for antineoplastic therapy. This also suggests that As2O3 can be used at a low concentration and still selectively target the microtubules of rapidly dividing tumor cells, thus minimizing its general toxicity. Evidence that tubulin plays an important role in the treatment of leukemia raises the possibility for the development of a rationally designed arsenic-based antimitotic agent.
Figures and Tables
 | Fig. 1Induction of cytotoxicity and cell cycle arrest by As2O3. (A) To show the inhibitory effects of As2O3 on the cell proliferation, exponentially growing cells were treated with the indicated concentrations of As2O3 for 24 hour and 48 hours, and cell proliferation was assessed by using the MTS reagent (see Materials and Methods). The cytotoxic effect of As2O3 was increased in a concentration- and/or time-dependent manner. These data represent the mean±SD of independent experiments. (B) To observe the apoptotic effects of As2O3, NB4 cells were treated with either a vehicle (control) or each concentration of As2O3 and paclitaxel for 24 hours. PI-stained cells were analyzed by flow cytometry as described in the Materials and Methods section, and the percentage of cells in each phase of the cell cycle was analyzed using flow cytometry (Table 1). Flow cytometry analysis revealed a significant increased apoptotic effect that resulted in a concentration-dependent accumulation of NB4 cells in the G2/M phase on the As2O3-treated cells. |
 | Fig. 2Alterations in tubulin polymerization induced by As2O3. (A) To demonstrate that the fraction of depolymerized tubulin is increased after treatment with As2O3, NB4 cells were treated with drugs or without drugs as a control for 6 hours at the indicated concentrations. Cell lysates were separated into polymerized (P) or soluble (S) fractions. Aliquots of equal volume were separated on an SDS-PAGE gel, and evaluated by western blotting with an anti α-tubulin antibody. Compared to the baseline proportion of α-tubulin in the soluble fraction, the depolymerized proportion observed after treatment of As2O3 was approximately 52-72% and the tubulin shift to the "S" fraction was noted. (B) To show the effects of a combination of drugs, NB4 cells were treated with 5 µM As2O3, 10 µM As2O3 and 40 nM paclitaxel at alone or in combination, and treated for 6 hours. Cell lysates were separated into "P" or "S" fractions as described above. Aliquots of equal volume were loaded onto SDS-PAGE gels, and the blots probed with antibody against α-tubulin. The results indicate that As2O3 and paclitaxel did not synergistically influence microtubule assembly. The intensity of each band was quantified by densitometry and the percentage of soluble tubulin (%S) was calculated by multiplying the fraction of tubulin in the soluble fraction [S/(S+P)] by 100 for each "S-P" pair. |
 | Fig. 3Effects of As2O3 on microtubule stabilization. NB4 cells were treated with drugs or no drugs as a control, for 6 hours and 24 hours at the indicated concentration. Lysates were separated into polymerized (P) or soluble (S) fractions. The blots probed with anti-acetylated α-tubulin. The amount of acetylated α-tubulin in the P fraction of paclitaxel treatment was increased, compared with that of the control, but the proportion in P fraction of As2O3 treatment was decreased in a concentration-dependent manner. This result indicated that As2O3 interfered with important modifications necessary for the stability of microtubules. The intensity of each band was quantified by densitometry and the blots were stripped and reprobed with GAPDH-specific antibody as a loading control. |
 | Fig. 4Effects of As2O3 on microtubule polymerization in vitro. Purified tubulin from bovine brain tissue (Cytoskeleton) was incubated at 37℃ in reaction mixtures containing 1 mM GTP, 10 nM paclitaxel (Taxol), 10 nM vincristine, 5 µM As2O3 and the mock-treated solution as a control. Tubulin polymerization was determined by measuring absorbance at 340 nm. This revealed that As2O3 directly influences tubulin and induces tubulin depolymerization. |
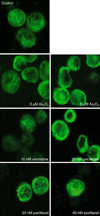 | Fig. 5Effects of As2O3 on the organization of cellular microtubule network. NB4 cells were treated with 5 µM As2O3, 40 nM paclitaxel, and 20 nM vincristine. Mock-treated cells were used as a control. After a 6-hour incubation, cells were harvested and fixed with formaldehyde. Cells were incubated with monoclonal anti-α-tubulin antibody at room temperature for 30 minutes. After incubation with FITC-conjugated secondary antibody, the cellular microtubules were imaged using an Olympus IX-81 fluorescence microscope. The normal organization of microtubule network was seen in control cells, increased density of polymerized microtubules were found in paclitaxel-treated cells, and a degraded microtubule network in cytoplasm was observed in As2O3-and vicristine-treated cells. |
References
1. Kamimura T, Miyamoto T, Harada M, Akashi K. Advances in therapies for acute promyelocytic leukemia. Cancer Sci. 2011. 102:1929–1937.

2. Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002. 62:3893–3903.
3. Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007. 18:Suppl 5. v3–v8.

4. Barlow SB, Gonzalez-Garay ML, Cabral F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J Cell Sci. 2002. 115:3469–3478.

5. Kavallaris M, Tait AS, Walsh BJ, et al. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001. 61:5803–5809.
6. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004. 4:253–265.

7. Huang SC, Lee TC. Arsenite inhibits mitotic division and perturbs spindle dynamics in HeLa S3 cells. Carcinogenesis. 1998. 19:889–896.

8. Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 2002. 62:529–538.

9. Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999. 59:776–780.
10. Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003. 9:660–664.

11. Poruchynsky MS, Kim JH, Nogales E, et al. Tumor cells resistant to a microtubule-depolymerizing hemiasterlin analogue, HTI-286, have mutations in alpha- or beta-tubulin and increased microtubule stability. Biochemistry. 2004. 43:13944–13954.

12. Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996. 88:1052–1061.

13. Carré M, Carles G, André N, et al. Involvement of microtubules and mitochondria in the antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem Pharmacol. 2002. 63:1831–1842.

14. Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003. 421:230.
15. Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000. 113:3907–3919.

16. Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005. 23:2726–2734.

17. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002. 2:48–58.

18. Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003. 22:7280–7295.

19. Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol Cancer Ther. 2003. 2:597–605.
20. Freitas RA, Silva dos, Gimenes Teixeira HL, et al. Apoptosis induction by (+)alpha-tocopheryl succinate in the absence or presence of all-trans retinoic acid and arsenic trioxide in NB4, NB4-R2 and primary APL cells. Leuk Res. 2009. 33:958–963.

21. Dong JT, Luo XM. Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res. 1993. 302:97–102.

22. Van Wijk R, Welters M, Souren JE, Ovelgonne H, Wiegant FA. Serum-stimulated cell cycle progression and stress protein synthesis in C3H10T1/2 fibroblasts treated with sodium arsenite. J Cell Physiol. 1993. 155:265–272.

23. Yih LH, Lee TC. Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res. 2000. 60:6346–6352.
24. Minotti AM, Barlow SB, Cabral F. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J Biol Chem. 1991. 266:3987–3994.

25. MacRae TH. Tubulin post-translational modifications-enzymes and their mechanisms of action. Eur J Biochem. 1997. 244:265–278.

26. Jiang JD, Wang Y, Roboz J, Strauchen J, Holland JF, Bekesi JG. Inhibition of microtubule assembly in tumor cells by 3-bromoacetylamino benzoylurea, a new cancericidal compound. Cancer Res. 1998. 58:2126–2133.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download