Abstract
Thrombotic thrombocytopenic purpura (TTP) is a critical complication of treatment with mitomycin C. We retrospectively describe the case of a patient with progressive renal cell carcinoma and mitomycin-induced TTP refractory to plasma exchange and glucocorticoids; we describe the clinical course, successful management of TTP with rituximab, and follow-up of this case. Mitomycin-induced TTP resolved completely by a total of 4 infusions of rituximab 375 mg/m2 on a weekly basis, and it took up to 12 months to obtain a platelet count of >100,000/µL. Rituximab is indicated for the treatment of mitomycin-induced TTP refractory to plasma exchange and glucocorticoids, and it could improve the patient's quality of life despite the presence of underlying malignancy.
Thrombotic thrombocytopenic purpura (TTP) is a syndrome characterized by microangiopathic hemolytic anemia, thrombocytopenia, acute renal insufficiency, neurologic abnormalities, and fever. It is diagnosed primarily by the presence of microangiopathic hemolytic anemia and thrombocytopenia [1]. Although many cases of TTP are idiopathic, a variety of causes have been identified. Some chemotherapeutic agents, including mitomycin C (MMC), have been involved in TTP, and direct endothelial injury is the initiating event in these settings [2]. Chemotherapeutic agent-induced TTP is often refractory to standard treatments such as plasma exchange. We present a case of mitomycin-induced TTP refractory to prolonged plasma exchange and methylprednisolone; the patient was successfully treated with rituximab, an anti-CD20 monoclonal antibody.
A 56-year-old man with renal cell carcinoma was admitted to our hospital for the evaluation of dyspnea and pretibial edema, which he had for 2 weeks. Renal cell carcinoma was diagnosed about 3 years ago when he had undergone left radical nephrectomy. Ten months after the nephrectomy, multiple hematogenous metastases had been found in both the lungs, and he had received salvage chemotherapy with aldesleukin, 5-fluorouracil (5-FU), interferon alfa-2b (IFN-α-2b), sorafenib, and MMC for 1 year; in particular, MMC 9 mg was administered intravenously 10 times over 3 months until 2 months before this admission.
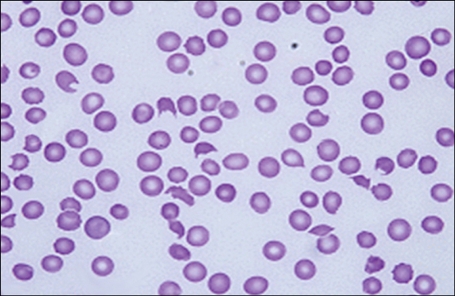
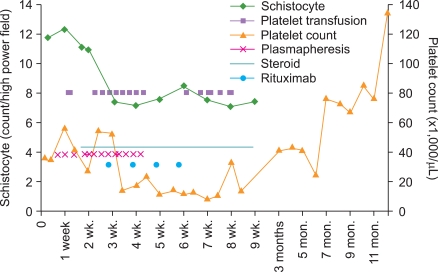
On admission, the patient's vital signs were stable; fever was absent; and he was alert and oriented. Laboratory data showed that the Hb level was 9.2 g/dL; platelet count, 36,000/µL; WBC count, 6,160/µL; blood urea nitrogen (BUN) level, 38.9 mg/dL; creatinine level, 2.4 mg/dL; international normalized ratio (INR, which indicated the prothrombin time [PT]), 1.26; activated partial thromboplastin time (aPTT), 33.5 sec; fibrinogen level, 411 mg/dL; and D-dimer level, 1.52 µg/mL. On hospital day (HD) 4, his dyspnea exacerbated, and he developed cough with blood-tinged sputum and fever with body temperature of up to 38.5℃. Laboratory data showed that the Hb level was 7.7 g/dL; platelet count, 27,000/µL; WBC count, 9,860/µL; BUN level, 32.2 mg/dL; creatinine level, 2.2 mg/dL; and lactic dehydrogenase (LDH) level, 629 IU/L; further, antinuclear and antineutrophil cytoplasmic antibodies (ANCA) were absent. A peripheral blood smear revealed a marked decrease in the number of platelets (no clumping was noted) and moderate degree of anisopoikilocytosis with a significant number of schistocytes and spherocytes per high power field (Fig. 1). On HD 5, he was intubated and ventilated because of respiratory failure resulting from pulmonary edema and hemorrhage. From the same day, he underwent daily plasmapheresis under the diagnosis of TTP; however, his clinical course did not improve. From HD 12, in addition to daily plasmapheresis, methylprednisolone 125 mg was administered intravenously twice a day because the patient did not respond to plasmapheresis alone. Laboratory data showed that the Hb level was 11.6 g/dL (with intermittent RBC transfusion); platelet count, 21,000/µL; WBC count, 10,400/µL; reticulocyte count, 3.79%; BUN level, 48.5 mg/dL; creatinine level, 3.4 mg/dL; LDH level, 1,075 IU/L; INR, 1.4; aPTT, 43.2 sec; fibrinogen level, 496 mg/dL; D-dimer level, 2.23 µg/mL; and haptoglobin level, <10 mg/dL. A blood smear revealed marked anisopoikilocytosis, with 11 schistocytes/high power field. During the next 8 days, he did not respond clinically to plasmapheresis plus methylprednisolone therapy. On HD 20, in addition to daily plasmapheresis and methylprednisolone, rituximab 375 mg/m2 was administered intravenously; thereafter, rituximab was administered 3 times more at weekly intervals (Fig. 2). Meanwhile, plasmapheresis was performed on alternate days, during HD 24-30; by HD 30, plasmapheresis was performed 19 times and was discontinued. On HD 25, the patient was extubated, and he has been mentally alert since then. Until HD 55, he required platelet transfusion almost every other day to maintain a platelet count of ≥10,000/µL; thereafter, the count was maintained above 10,000/µL without further platelet transfusion. Moreover, until HD 29, RBC was transfused almost every other day to maintain a Hb level of >8 g/dL, but subsequently, the RBC transfusion interval was progressively extended until transfusion was performed once a week. The LDH level normalized 5 months after rituximab administration.
The creatinine level progressively increased up to 7.0 mg/dL during the 12-month follow-up period. This progressive renal dysfunction was likely to be associated with underlying diseases such as renal cell carcinoma, diabetes mellitus, hypertension, and drug toxicity. On HD 74, prednisolone therapy was completed, and on the next day he was discharged in good general condition.
At present, 12 months after the event, his general condition is fairly good (Eastern Cooperative Oncology Group [ECOG] score, 1), even though the metastatic renal cell carcinoma has advanced. His Hb level and platelet count are 11.7 g/dL and 111,000/µL, respectively, and creatinine level is 7.0 mg/dL.
MMC has antitumor antibiotic activity and is used as a chemotherapeutic agent. It is an alkylating agent, which produces DNA cross-links and inhibits DNA and RNA synthesis. It has been used to treat a variety of cancers, including gastrointestinal, breast, and bladder cancers. MMC-induced TTP-hemolytic uremic syndrome (HUS) may develop within weeks to months of the last dose of MMC, and the overall incidence is related to the cumulative dose [3]. The risk of developing TTP-HUS after treatment with MMC is 4-15% [4, 5].
The only requirement for making an initial diagnosis of idiopathic TTP-HUS and initiating treatment with plasma exchange is the presence of thrombocytopenia and microangiopathic hemolytic anemia [1]. Plasma exchange is the most effective treatment for TTP-HUS, and it reduces the mortality rate to 13-22% [6]. However, 10-20% of patients show a transient, incomplete, or no response to plasma exchange. Rituximab should be administered in addition to plasma exchange in cases of refractory or recurrent TTP-HUS. Scully et al. reported that 25 patients with acute refractory or relapsing idiopathic TTP received rituximab in conjunction with plasma exchange [7]. All the patients attained complete remission (CR) in a median of 11 days after rituximab administration was initiated. Among the cases of acute refractory TTP, the median numbers of plasma exchange performed before rituximab administration and after the first rituximab infusion were 13 and 9, respectively.
The prognosis for patients with MMC-induced TTP is poor. Most patients die within 4 months of diagnosis, either from pulmonary or renal failure or from the underlying malignancies [3, 8]. Plasma exchange is ineffective in most patients, and no effective therapy has yet been identified. Only a few cases have reported that MMC-induced TTP has been successfully treated with vincristine and cyclophosphamide therapy and staphylococcal protein A immunoadsorption therapy [10, 11]. In addition, one case reported successful treatment of MMC-induced TTP with rituximab [11].
Our patient presented with dyspnea associated with pulmonary edema, which progressed to adult respiratory distress syndrome (ARDS), as occurs in many patients with MMC-induced TTP [3, 8]. In our case, the cumulative dose of mitomycin was 87 mg. A previous report mentions that in a majority of cases, mitomycin-related TTP-HUS developed when the patient received a cumulative dose of ≥60 mg, and 58% of the cases had received doses of 60-100 mg [3]. Our patient was refractory to daily plasmapheresis and concurrent high-dose prednisolone for 16 days; however, he began to respond slowly to rituximab 375 mg/m2; a total of 4 doses of rituximab were administered on a weekly basis. ARDS was treated soon after the initiation of rituximab; it took 5 weeks to maintain a platelet count of ≥10,000/µL without platelet transfusion. The patient achieved clinical CR, defined by Elliott et al., almost 12 months from the date of the first dose of rituximab; the normal platelet count was maintained for at least 30 days, with the absence of clinical manifestations of TTP [12]. Elliott et al. reported that the median number of days to achieve CR from the date of the first dose of rituximab ranged from 11 days to 35 days across various studies for refractory or relapsing TTP related to ADAMTS13-deficiency.
When his renal cell carcinoma had metastasized to both lungs, our patient had received salvage chemotherapy with aldesleukin, 5-FU, IFN-α-2b, sorafenib, and MMC. Among the anticancer chemotherapeutic agents, MMC and 5-FU cause TTP [3]. A dose-response pattern was noted between MMC and TTP. However, 5-FU did not reveal a similar dose-response pattern, which suggested that 5-FU did not have a role in the development of TTP. TTP could develop after a few months to several years of IFN-α therapy for chronic myeloid leukemia (CML) or chronic hepatitis C [13-15]. IFN-α-induced TTP responds well to plasma exchange, and the patient recovers spontaneously after discontinuation of IFN-α. Therefore, we assumed that TTP in our case was attributed to MMC.
To our knowledge, only a single case has been reported in which MMC-induced TTP refractory to plasma exchange and prednisone was successfully and promptly treated with rituximab [11]. In contrast to that reported in the previous case, our patient showed slow response to rituximab treatment but ended up achieving clinical CR despite progressive underlying malignancy.
References
1. George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000; 96:1223–1229. PMID: 10942361.

2. Groff JA, Kozak M, Boehmer JP, Demko TM, Diamond JR. Endotheliopathy: a continuum of hemolytic uremic syndrome due to mitomycin therapy. Am J Kidney Dis. 1997; 29:280–284. PMID: 9016902.

3. Lesesne JB, Rothschild N, Erickson B, et al. Cancer-associated hemolytic-uremic syndrome: analysis of 85 cases from a national registry. J Clin Oncol. 1989; 7:781–789. PMID: 2497229.

4. Proia AD, Harden EA, Silberman HR. Mitomycin-induced hemolytic-uremic syndrome. Arch Pathol Lab Med. 1984; 108:959–962. PMID: 6210069.
5. Valavaara R, Nordman E. Renal complications of mitomycin C therapy with special reference to the total dose. Cancer. 1985; 55:47–50. PMID: 3917353.

6. von Baeyer H. Plasmapheresis in thrombotic microangiopathy-associated syndromes: review of outcome data derived from clinical trials and open studies. Ther Apher. 2002; 6:320–328. PMID: 12164804.

7. Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 2007; 136:451–461. PMID: 17233847.

8. Cantrell JE Jr, Phillips TM, Schein PS. Carcinoma-associated hemolytic-uremic syndrome: a complication of mitomycin C chemotherapy. J Clin Oncol. 1985; 3:723–734. PMID: 3923162.

9. Durand JM, Lefévre P. Mitomycin-induced thrombotic thrombocytopenic purpura: possible successful treatment with vincristine and cyclophosphamide. Haematologica. 1991; 76:421–423. PMID: 1839627.
10. Kasper S, Neurath MF, Huber C, Theobald M, Scharrer I. Protein A immunoadsorption therapy for refractory, mitomycin C-associated thrombotic microangiopathy. Transfusion. 2007; 47:1263–1267. PMID: 17581162.
11. Onitilo AA, Engel JM, Clouse LH, Gerndt KM. Successful treatment of mitomycin-induced thrombotic thrombocytopenic purpura with rituximab. J Vasc Interv Radiol. 2009; 20:275–276. PMID: 19054690.

12. Elliott MA, Heit JA, Pruthi RK, Gastineau DA, Winters JL, Hook CC. Rituximab for refractory and or relapsing thrombotic thrombocytopenic purpura related to immune-mediated severe ADAMTS13-deficiency: a report of four cases and a systematic review of the literature. Eur J Haematol. 2009; 83:365–372. PMID: 19508684.

13. Al-Zahrani H, Gupta V, Minden MD, Messner HA, Lipton JH. Vascular events associated with alpha interferon therapy. Leuk Lymphoma. 2003; 44:471–475. PMID: 12688317.

14. Rachmani R, Avigdor A, Youkla M, et al. Thrombotic thrombocytopenic purpura complicating chronic myelogenous leukemia treated with interferon-alpha. A report of two successfully treated patients. Acta Haematol. 1998; 100:204–206. PMID: 9973644.
15. Kitano K, Gibo Y, Kamijo A, et al. Thrombotic thrombocytopenic purpura associated with pegylated-interferon alpha-2a by an ADAMTS13 inhibitor in a patient with chronic hepatitis C. Haematologica. 2006; 91(8 Suppl):ECR34. PMID: 16923518.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download