Abstract
Background
Intraoperative cell salvage exerts shear stress upon RBCs, particularly as they are suctioned from the surgical field. Shear stress can result in overt hemolysis or it can cause sublethal injury to the suctioned RBCs. The mechanical fragility (MF) test uses shear stress to measure the extent of RBC sublethal injury. RBCs that have sustained sublethal injury are more susceptible to shear stress induced hemolysis. In this study we suctioned whole blood samples from an artificial surgical field to determine if pre-menopausal female RBCs would demonstrate greater resistance to hemolysis and less sublethal injury compared to that of males and post-menopausal females.
Methods
Ten CPD-preserved whole blood units from these 3 donor groups were obtained and samples suctioned at -150 mmHg from a simulated surgical field. The MF test was then performed and the % hemolysis calculated. In addition the MF test was serially performed on these whole blood units during the 21 days of storage.
Results
There were no differences in the extent of hemolysis or RBC shear stress resistance after suctioning between the 3 donor groups. During storage the pre-menopausal female RBCs demonstrated higher shear stress tolerance compared to the males or post-menopausal females at all of the time points.
Intraoperative cell salvage (ICS) is a process in which whole blood from the surgical field is suctioned, processed, washed, and the red blood cells (RBC) are returned to the patient. Throughout this process, but particularly during suctioning, mechanical stress is applied to the RBCs [1, 2]. A mathematical model of ICS has predicted that small improvements in RBC processing efficiency can result in significantly greater amounts of recovered RBCs. With the same mathematical model, real cases were matched to the model which revealed that wide differences in processing efficiency occurred from case to case despite very similar case management [3]. The discrepancy in RBC recovery between the mathematical model and actual patient outcomes suggests that the RBC capture and return efficiency of ICS is highly variable between patients. This variability might be caused in part by patient-specific factors, such as intrinsic differences in RBC shear stress tolerance.
The mechanical fragility (MF) test is a reliable and reproducible method of applying shear stress to RBCs. The cells that were intact at the beginning of the assay but that lyse once the shear stress is applied are cells that featured reduced shear stress tolerance. The output of the MF test is the Mechanical Fragility index (MFI); higher MFI values indicate that the RBCs are more predisposed to lysis when exposed to shear stress.
We have previously used the MFI to evaluate the changes that occurred during storage in AS-5 and SAGM preserved RBCs from male, pre-menopausal female, and post-menopausal female donors [4, 5]. Throughout storage the pre-menopausal female RBCs demonstrated lower MFI values (greater resistance to shear stress induced hemolysis) in both preservative solutions compared to the other 2 donor categories. Thus there appear to be intrinsic differences in shear stress tolerance between the RBCs from pre-menopausal females and the RBCs from both post-menopausal females and males. Since pre-menopausal female RBCs stored in additive solutions demonstrated greater shear stress tolerance during storage, we asked the question, do pre-menopausal female RBCs demonstrate less hemolysis and have a lower MFI after being suctioned from an artificial surgical field relative to male and post-menopausal female RBCs? We also serially measured the MFI from CPD-preserved whole blood units from these 3 donor groups during storage.
From each of the following 3 donor demographics, 10 CPD-preserved whole blood units were obtained (total of 30 units) from the regional FDA-licensed blood center on day 3 post-collection: males, pre-menopausal females, and post-menopausal females. Day 3 post-collection was the earliest time at which whole blood units which had passed the transmissible disease screening tests were available.
After thorough mixing, 60 mL aliquots from each of the CPD-preserved whole blood units were adjusted to a standard hematocrit of 30% using Dulbecco's PBS (Lonza BioWhittaker DPBS with Calcium and Magnesium, Fisher Scientific, Pittsburgh, PA, USA) and the hemoglobin (Hb) concentration of each aliquot was measured (ABX Micros 60 Hematology Analyzer, Horiba ABX Inc., Irvine, CA, USA). Forty mL of blood was then applied to a flat metal tray as previously described [6]. Briefly, the whole blood was suctioned at a constant pressure of -150 mmHg using a surgical suction pump with a Yankauer suction catheter tip (Medi-Vac Yankauer suction handle, Catalog K87, Cardinal Health, McGaw Park, IL, USA). The catheter was connected to a standard collection canister via standard suction tubing. The MF test (described below) was then performed on each aliquot of suctioned whole blood. The remaining blood from the diluted aliquot from each unit was not suctioned and thus served as the MF test control.
CPD-preserved whole blood units were stored between 1-6℃ for 21 days. On storage days 3, 7, 14, and 21, 60 mL aliquots from each whole blood unit were adjusted to a standard hematocrit of 30% using Dulbecco's PBS and the hemoglobin concentration was measured. The MF test was then performed on each aliquot.
The MF test was performed as described in reference [4]. Briefly, 3 mL from each sample to be tested was added to 5 test tubes. Three of these test-tubes contained five 3.2 mm stainless steel ball bearings (BB) and were rocked for 1 hour on a rocking platform (Type M79700 Platform Vari-Mix rocker; Barnstead Thermolyne Corp., Dubuque, IA, USA) at an angle of ±17° from the horizontal at a rate of 18 cycles/min. The remaining 2 tubes were not rocked and did not contain BBs; they served as controls to determine the baseline level of free Hb. After 1 hour, the RBCs from both the rocked and unrocked samples were transferred to new tubes without BBs and were centrifuged twice to ensure the removal of membrane fragments and debris. The supernatant was decanted and the free Hb concentration was measured by the plasma light absorbance at 540 nm (Spectronic Genesys 5 spectrophotometer; Spectronic Instruments, Inc., Columbus, OH, USA). The mechanical fragility index (MFI) and quantitation of hemolysis were calculated as follows:
MFI=[(fHbrocked-fHbunrocked)/(Hbaliquot-fHbunrocked)]×100 % hemolysis=[(100-hematocrit)×(fHbunrocked)]/Hbaliquot
Where fHbrocked is the average free Hb concentration in supernatant of rocked samples, fHbunrocked is the average free Hb concentration in supernatant of unrocked samples, and Hbaliquot is the Hb concentration of the 60 mL RBC aliquot after dilution to 30% hematocrit.
For all whole blood samples, the average donor ages, the average pre- and post-suctioning MFI, and the average pre- and post-suctioning percent hemolysis were compared with one- and two-way analysis of variance. Post hoc analysis utilizing Bonferroni's multiple comparison test was implemented as needed. Results are presented as mean (±SD). Differences were considered statistically significant at P<0.05.
Ten RBCs from each of the donor demographics were studied. The mean age of the male, pre-menopausal female, and post-menopausal female donors was 46.2 years (±12.8), 25.2 years (±6.3), and 62.6 years (±6.1), respectively. The pre-menopausal females were significantly younger than both the post-menopausal females (P<0.001) and the males (P<0.001). The post-menopausal females were significantly older than the male donors (P>0.01).
For all 3 donor populations, the mean post-suctioning MFI was not significantly different than the mean pre-suctioning MFI (P>0.05) (Fig. 1). However, the pre-menopausal female RBCs demonstrated lower pre- and post-suctioning mean MFI values compared to both the post-menopausal female and male RBCs (P<0.05 or less for all comparisons). In all 3 donor populations, the mean post-suction % hemolysis was significantly greater than the mean pre-suction % hemolysis (P<0.001 for all 3 donor groups) (Fig. 2). The pre-suction % hemolysis values did not differ significantly between the 3 groups. Likewise the post-suction % hemolysis values did not differ significantly between the 3 groups.
The MFI of the RBCs from all 3 donor groups increased significantly between storage day 3 and 21 (Fig. 3; P<0.001 for all 3 donor groups). The MFI of the pre-menopausal females was significantly lower than that of both the males (P<0.001, at all of the time points) and post-menopausal females (P<0.01 at days 3 and 7, P<0.001 at days 14 and 21). At all of the time points there was no statistically significant difference in the mean MFI of the male and post-menopausal female RBCs. The mean % hemolysis from all 3 donor populations was less than 1% at day 21 (Fig. 4), and there was no statistically significant increase in the % hemolysis between any 2 time points during this study for all 3 donor groups.
In this study, the same degree of hemolysis was seen in all 3 donor populations after suctioning. This indicates that pre-menopausal female RBCs in whole blood did not demonstrate enhanced tolerance of suctioning compared to male or post-menopausal female RBCs. These findings suggest that RBCs from all of the groups are equally affected by the stresses produced by suctioning. In a previous study, similar trends in the MFI and % hemolysis were seen in the pre- and post-suctioning samples of reconstituted whole blood which was suctioned from an artificial surgical surface [6], although the age and gender of the donors in the earlier study were not reported.
It has been demonstrated that both gender and menopausal status affect the MFI of red cell concentrates in two different additive solutions [4, 5]. Male and post-menopausal female RBCs have MFIs that were significantly higher compared to those of pre-menopausal female RBCs, indicating that the RBCs of pre-menopausal women had a higher resistance to mechanical stress compared to blood from the other 2 donor groups. These differences were again present in this study as the pre-menopausal females demonstrated the lowest MFI levels both pre- and post-suctioning, as well as throughout storage. The reasons for the pre-menopausal female RBCs' higher shear stress resistance are unclear but could relate to an overall younger age of their RBCs caused by menstrual bleeding, or hormonal factors that are absent from post-menopausal females and males.
The serial analysis of MFI and % hemolysis in the whole blood units stored under routine blood bank conditions demonstrated a smaller absolute increase in MFI over the 21-day storage interval compared to red cell concentrates stored in either AS-5 or SAGM over the first 21 days of storage [4, 5]. This is possibly due to the much larger quantity of plasma proteins present in whole blood units compared to the red cell concentrates; SAGM RBCs contain approximately 10-15 mL of plasma and AS-5 RBCs contain 30-50 mL of plasma, while whole blood units contain approximately 300 mL of plasma. Plasma proteins stabilize the RBC membrane when undergoing shear stress which might serve to decrease the MFI [7].
Given that pre-menopausal female RBCs demonstrated enhanced shear stress tolerance during storage in 2 different RBC preservative solutions [4, 5], and in whole blood (Fig. 3), it was hypothesized that their RBCs would also demonstrate less hemolysis after suctioning. Indeed this was not the case as the post-suctioning % hemolysis was not statistically different between the 3 donor groups, which suggests that the stresses produced during suctioning overwhelm the increased shear stress resistance (i.e., lower MFI) that has been found in pre-menopausal female RBCs. The cells that remained after suctioning demonstrated very slightly reduced MFI values compared to the pre-suctioning cohort for all 3 donor groups. Perhaps this is because the most fragile RBCs were lysed during suctioning, while the remaining intact RBCs were less affected by the suctioning forces.
The limitations of this study include the fact that our in vitro model replicates only the suctioning step in the ICS process - centrifugation, washing, and warming steps which occur after suctioning in an actual surgical case were not incorporated in our experimental design and might contribute more damage to the RBCs. In addition it is possible that during a long surgery the same RBC might be suctioned and processed multiple times, thus potentially multiplying the sublethal injury sustained. Furthermore, during an actual surgery the patient could be exposed to numerous lipid soluble medications that, if intercalated into the RBC membrane, might alter their shear stress tolerance. The significance of these changes in MFI and % hemolysis to cell salvage recipients need to be assessed in clinical studies.
In conclusion, although during static storage in the blood bank the RBCs from pre-menopausal females demonstrate greater shear stress tolerance than that from post-menopausal females and males, this advantage does not seem to reduce the extent of hemolysis that occurs when RBCs are suctioned by a cell salvage device.
References
1. Gregoretti S. Suction-induced hemolysis at various vacuum pressures: implications for intraoperative blood salvage. Transfusion. 1996; 36:57–60. PMID: 8607155.

2. Waters JH, Williams B, Yazer MH, Kameneva MV. Modification of suction-induced hemolysis during cell salvage. Anesth Analg. 2007; 104:684–687. PMID: 17312230.

3. Waters JH, Lee JS, Karafa MT. A mathematical model of cell salvage efficiency. Anesth Analg. 2002; 95:1312–1317. PMID: 12401618.

4. Raval JS, Waters JH, Seltsam A, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010; 99:325–331. PMID: 20673245.

5. Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2010; (published online http://onlinelibrary.wiley.com/doi/10.1111/j.1423-0410.2010.01439.x/full).

6. Yazer MH, Waters JH, Elkin KR, Rohrbaugh ME, Kameneva MV. A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion. 2008; 48:1188–1191. PMID: 18346016.

7. Kameneva MV, Antaki JF, Yeleswarapu KK, Watach MJ, Griffith BP, Borovetz HS. Plasma protective effect on red blood cells exposed to mechanical stress. ASAIO J. 1997; 43:M571–M575. PMID: 9360109.

Fig. 1
The mean MFI of RBCs in CPD-preserved whole blood from each donor group before and after suctioning. Ten whole blood units from each of these 3 donor demographics were tested. For all 3 donor groups there was no statistically significant change in the MFI after suctioning. The pre-menopausal female RBCs demonstrated lower pre-suctioning MFI values compared to the post-menopausal female RBCs (P<0.01) and the male RBCs (P<0.001). Likewise, the pre-menopausal female RBCs demonstrated lower post-suctioning MFI values compared to the post-menopausal female RBCs (P<0.05) and the male RBCs (P<0.001). The error bars represent 1 SD.
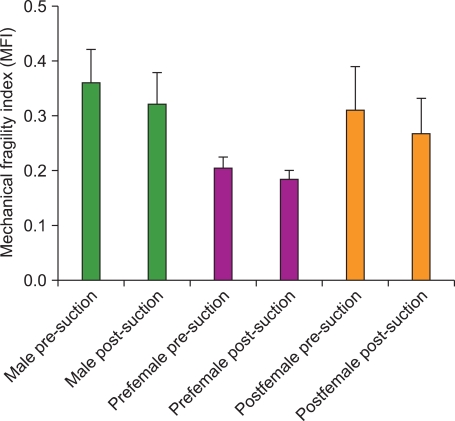
Fig. 2
The mean % hemolysis of RBCs in CPD-preserved whole blood from each donor group before and after suctioning. Ten whole blood units from each of these 3 donor demographics were tested. For all 3 donors groups there was a statistically significant increase in the % hemolysis after suctioning. The pre-suction % hemolysis values did not differ significantly between the 3 groups. Likewise the post-suction % hemolysis values did not differ significantly between the 3 groups. The error bars represent 1 SD.
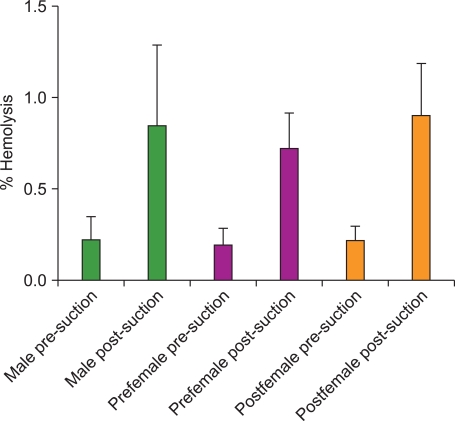
Fig. 3
The mean MFI of RBCs in CPD-preserved whole blood from the 3 donor groups measured serially over 21-days of storage. Ten whole blood units from each of these 3 donor demographics were tested. The MFI of the pre-menopausal females was significantly lower than that of both the males at all of the time points (P<0.001) and post-menopausal females (P<0.01 at days 3 and 7, P<0.001 at days 14 and 21).

Fig. 4
The mean % hemolysis in CPD-preserved whole blood units from the 3 donor groups measured serially over 21-days of storage. Ten whole blood units from each of these 3 donor demographics were tested. The increase in % hemolysis over time was not significant for any of the 3 donor groups, nor was the difference in the % hemolysis at any of the time points significantly different between the 3 donor groups.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download