1. Ringden O, Remberger M, Svahn BM, et al. Allogeneic hematopoietic stem cell transplantation for inherited disorders: experience in a single center. Transplantation. 2006; 81:718–725. PMID:
16534474.
2. Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003; 31:229–239. PMID:
12621457.

3. Srinivasan R, Takahashi Y, McCoy JP, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006; 133:305–314. PMID:
16643433.

4. McCann SR, Bacigalupo A, Gluckman E, et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant. 1994; 13:233–237. PMID:
8199566.
5. Bacigalupo A, Brand R, Oneto R, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy-The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000; 37:69–80. PMID:
10676912.

6. Lawler M, McCann SR, Marsh JC, et al. Serial chimerism analyses indicate that mixed haemopoietic chimerism influences the probability of graft rejection and disease recurrence following allogeneic stem cell transplantation (SCT) for severe aplastic anaemia (SAA): indication for routine assessment of chimerism post SCT for SAA. Br J Haematol. 2009; 144:933–945. PMID:
19183198.

7. Hoelle W, Beck JF, Dueckers G, et al. Clinical relevance of serial quantitative analysis of hematopoietic chimerism after allogeneic stem cell transplantation in children for severe aplastic anemia. Bone Marrow Transplant. 2004; 33:219–223. PMID:
14647253.

8. Willasch A, Hoelle W, Kreyenberg H, et al. Outcome of allogeneic stem cell transplantation in children with non-malignant diseases. Haematologica. 2006; 91:788–794. PMID:
16769581.
9. Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991; 324:667–674. PMID:
1994250.

10. Nollet F, Billiet J, Selleslag D, Criel A. Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transplant. 2001; 28:511–518. PMID:
11593326.

11. Svenberg P, Mattsson J, Ringdén O, Uzunel M. Allogeneic hematopoietic SCT in patients with non-malignant diseases, and importance of chimerism. Bone Marrow Transplant. 2009; 44:757–763. PMID:
19421178.

12. Boelens JJ, Wynn RF, O'Meara A, et al. Outcomes of hematopoietic stem cell transplantation for Hurlers syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant. 2007; 40:225–233. PMID:
17529997.

13. Ozyurek E, Cowan MJ, Koerper MA, Baxter-Lowe LA, Dvorak CC, Horn BN. Increasing mixed chimerism and the risk of graft loss in children undergoing allogeneic hematopoietic stem cell transplantation for non-malignant disorders. Bone Marrow Transplant. 2008; 42:83–91. PMID:
18391990.

14. Roy DC, Tantravahi R, Murray C, et al. Natural history of mixed chimerism after bone marrow transplantation with CD6-depleted allogeneic marrow: a stable equilibrium. Blood. 1990; 75:296–304. PMID:
1967216.

15. Baron F, Little MT, Storb R. Kinetics of engraftment following allogeneic hematopoietic cell transplantation with reducedintensity or nonmyeloablative conditioning. Blood Rev. 2005; 19:153–164. PMID:
15748963.

16. Sugita J, Tanaka J, Hashimoto A, et al. Influence of conditioning regimens and stem cell sources on donor-type chimerism early after stem cell transplantation. Ann Hematol. 2008; 87:1003–1008. PMID:
18636260.

17. Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005; 19:822–828. PMID:
15772701.

18. Mickelson DM, Sproat L, Dean R, et al. Comparison of donor chimerism following myeloablative and nonmyeloablative allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011; 46:84–89. PMID:
20305699.

19. Gonzalez-Vicent M, Diaz MA. Higher doses of CD34+ PBPC are associated with a rapid acquisition of full donor chimerism and lower risk of relapse after allogeneic transplantation in pediatric patients with hematological malignancies. J Pediatr Hematol Oncol. 2011; 33:185–189. PMID:
21325973.

20. Remberger M, Jaksch M, Uzunel M, Mattsson J. Serum levels of cytokines correlate to donor chimerism and acute graft-vs.-host disease after haematopoietic stem cell transplantation. Eur J Haematol. 2003; 70:384–391. PMID:
12756021.

21. Jillella AP, Shafer D, Klumpp TR, Emmons RV, Mangan KF. Mixed chimerism and graft failure following conditioning with the fludarabine and cyclophosphamide nonablative regimen; conversion to full donor chimerism. Am J Hematol. 2007; 82:419–426. PMID:
17211845.

22. Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007; 109:4586–4588. PMID:
17234738.

23. Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002; 29:987–989. PMID:
12098067.

24. Kim YR, Kim JS, Cheong JW, Song JW, Min YH. Transfusionassociated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol. 2008; 120:182–189. PMID:
19129689.

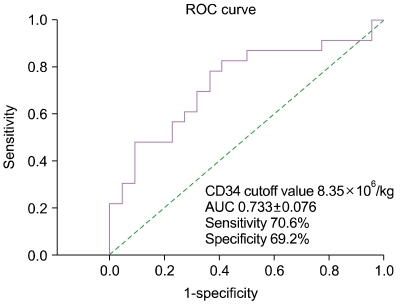
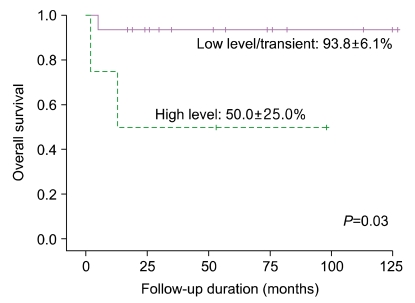
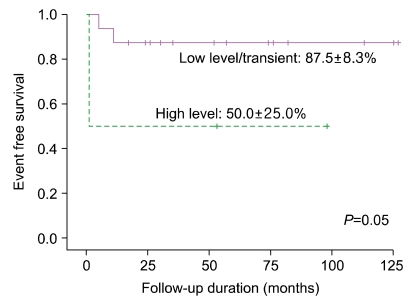
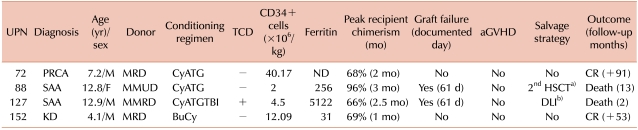




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download