Abstract
Background
Antagonists of CXC chemokine receptor 4 (CXCR4), including AMD3100, induce peripheral mobilization of hematopoietic stem cells and have been approved for clinical use. We explored whether the CXCR4 antagonists affected the survival and proliferation of myeloid leukemia cells in vitro.
Methods
The effects of CXCR4 antagonists AMD3100 and T140 on the survival and proliferation of myeloid leukemia cell lines (U937, HL-60, MO7e, KG1a, and K562) as well as CD34+ cells obtained from patients with AML and CML were analyzed by flow cytometry by using annexin V and a colorimetric cell proliferation assay.
Results
AMD3100, but not T140, stimulated the proliferation of leukemia cells in vitro in a dose-dependent manner for up to 5 days (~2-fold increase at a concentration of 10-5 M), which was not abrogated by pretreatment of the cells with pertussis toxin, but was attenuated by RNAi knockdown of CXCR7 transcripts. In contrast, AMD3100 induced a marked decrease in the cell numbers after 5-7 days. AMD3100, but not T140, induced phosphorylation of MAPK p44/p42. AMD3100 increased the number and size of leukemia cell colonies and reduced cell apoptosis during the first 5-7 days of incubation, but the phenomena were reversed during the later period of incubation.
The chemokine stromal cell-derived factor-1 (SDF-1) induces the migration and homing of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) by signaling via a G protein-coupled receptor, CXC chemokine receptor 4 (CXCR4) [1]. AML and CML cells also express CXCR4 [2,3] and respond to SDF-1, resulting in the trafficking of these cells in the bone marrow (BM) microenvironment [4]. SDF-1 alone has negligible effects on the proliferation of both normal and malignant hematopoietic cells in vitro [5], but the SDF-1/CXCR4 axis has been shown to be involved in the development and progression of myeloid leukemia. For example, AML patients with high expression levels of CXCR4 in CD34+ cells had a significantly reduced survival rate and a higher probability of relapse than their counterparts [6]. Human AML cells were shown to constitutively express SDF-1-dependent cell-surface elastase, which regulates their migration and proliferation [7]. A polymorphism in the SDF-1 gene has been correlated with the risk of distant tissue infiltration by AML cells [8], and functional CXCR4-expressing microparticles and SDF-1 expression were found to be correlated with circulating AML cells [9]. Compared to Philadelphia chromosome (Ph)-negative CD34+CXCR4+ cells, Ph-positive CD34+CXCR4+ cells from CML patients were shown to migrate poorly [10]. These observations suggest that the modulation of the SDF-1/CXCR4 axis may influence the biology of myeloid leukemia cells [11].
AMD3100, a small bicyclam molecule, was originally developed as a CXCR4 antagonist that blocked the entry of HIV into T cells [12]. AMD3100 inhibits the binding of SDF-1 to CXCR4 and induces peripheral mobilization of HSCs and HPCs [13]. AMD3100 induces the segregation of leukemic cells in the BM microenvironment [14, 15], resulting in enhanced chemosensitivity of the cells. On the basis of these observations, AMD3100 can be considered suitable for clinical application [11]. However, AMD3100 has been shown to activate a G protein coupled to CXCR4, and thus acts as a partial CXCR4 agonist in vitro [16]. Furthermore, AMD3100 was shown to exert dual effects in bleomycin-induced lung inflammation in an animal model [17]. We have previously reported that AMD3100 enhanced the survival and proliferation of myeloma cells in short-term incubation in vitro [18]. In the present study, we explored whether AMD3100 and another CXCR4 antagonist, T140, affected the survival and proliferation of myeloid leukemia cells in vitro.
BM samples were obtained, with informed consent, from 3 patients each with AML and CML at the time of diagnosis. CD34+ cells were purified from the BM by using the MACS system (Miltenyi Biotec, Auburn, CA). The human myeloid cell lines, U937, HL-60, K562, and KG1a, were purchased from the American Type Culture Collection (Manassas, VA). U937 cells were cultured in RPMI-1640 medium (Gibco-BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL Life Technologies). K562, KG1a, and HL-60 cells were grown in Isocove's modified Dulbecco's medium (IMDM; Gibco-BRL Life Technologies) supplemented with 10% FBS. MO7e cells were grown in IMDM supplemented with 10% FBS and 10 ng/mL granulocyte, macrophage colony-stimulating factor (R&D Systems, Minneapolis, MN). AMD3100 and pertussis toxin (PTX) were from Sigma Chemical Co. (St. Louis, MO). Another CXCR4 antagonist, the T140 peptide (H-Arg-Arg-Nal-Cys-Try-Arg-Lys-d Lys-Pro-Tyr-Arg-Cit-Cys-Arg-OH), was purchased from Peptron Inc. (Daejeon, Korea). Signal-blocking agents (wortmannin, LY294002, AG490, PD98059, rapamycin, and SB203580) were obtained from Calbiotech (Spring Valley, CA).
Cells were incubated with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated monoclonal antibodies at 4℃ for 30 min and analyzed using a Coulter Elite flow cytometer (Coulter Electronics Ltd., Hialeah, FL) or a FACSCanto II flow cytometer (BD Pharmingen, San Diego, CA). The monoclonal antibodies used were FITC-conjugated anti-CXCR4, PE-conjugated anti-CXCR4 (clone 12G5; BD Pharmingen), and APC-conjugated anti-CXCR7/RDC-1 (clone 11G8; R&D Systems). To detect cytoplasmic CXCR4 or CXCR7, the cells were permeabilized with a saponin-based reagent (BD Pharmingen) and labeled. To detect apoptosis, the cells were stained with FITC-conjugated annexin V (BD Pharmingen) and analyzed by flow cytometry. For cell cycle analysis, the cells were stained with propidium iodide (PI; BD Pharmingen) and analyzed.
For the transmigration experiments, cells (2×105 cells/well) were loaded into the upper chamber of a 24-well Transwell plate containing a 5-µm microporous membrane (Corning-Costar, Cambridge, MA), and the cells were allowed to migrate into the lower chamber containing 200 ng/mL SDF-1 for 4 hr.
To examine whether AMD3100 and T140 affected the proliferation of leukemia cells, the 5 leukemia cells and primary CD34+ cells from 3 patients were incubated in serum-free X-VIVO medium (BioWhittaker, Walkersville, MD) in the absence or presence of AMD3100 (10-7 M to 10-5 M) or T140 (10-7 M to 10-5 M), for up to 72 hr and analyzed using a colorimetric assay kit (CCK-8 assay kit; Dojindo Laboratories, Tokyo, Japan) according to the manufacturer's instructions. Briefly, 5×103 cells were incubated in 96-well plates in serum-free X-VIVO medium. After incubation, 10 µL of CCK-8 solution, which was provided by the manufacturer, was added to each well. The optical density (OD) was measured 3 hr later by using a spectrophotometer (Molecular Devices Co., Sunnyvale, CA). The proliferation index and relative proliferation index represent the fold-increase in the OD compared with that of the matched control at each incubation time point and the fold-increase in the OD compared with that of the control at the beginning of the incubation, respectively.
Cells (8×103) were plated in triplicate in 35-mm tissue culture dishes (Corning Costar), containing 1 mL of an assay medium consisting of X-VIVO medium and 1.2% methyl-cellulose (Stem Cell Technologies, Vancouver, BC, Canada). After 3-14 days of incubation at 37℃ in 5% CO2, the number of colonies, defined as cell clusters consisting of more than 5 cells, was counted using an inverted microscope.
Western blot was used to detect the phosphorylation of signaling molecules. Cells were starved in serum-free medium for 12 hr and then treated with AMD3100. The cells were collected by centrifugation, washed in phosphate-buffered saline, and lysed using SDS sample buffer (187.5 mM Tris-HCl, pH 6.8, 6% [w/v] SDS, 100% glycerol, 150 mM DTT, and 0.03% [w/v] bromophenol blue). Equal amounts of protein from each sample were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Amersham Life Science, Arlington Heights, IL). The membranes were blocked for 1 hr in TBS (Tris-buffered saline) containing 5% (w/v) milk and 0.1% Tween 20, and then incubated with a primary mouse or rabbit monoclonal antibody (Cell Signaling Technology Inc., Danvers, MA) overnight at 4℃. The blots were washed with TBS containing Tween 20, incubated with anti-mouse or anti-rabbit secondary antibody for 2 hr, and developed using West-Zol Plus (iNtRON Biotechnology, Seoul, Korea). The following antibodies were used: anti-phospho-Akt polyclonal antibody (Ser473), anti-Akt polyclonal antibody, anti-phospho-MAPK p44/p42 polyclonal antibody (Thr202, Tyr204), anti-MAPK p44/42 polyclonal antibody, anti-phospho-Stat3 polyclonal antibody (Tyr705), anti-Stat3 polyclonal antibody, anti-phospho-MAPK p38 polyclonal antibody (Thr180, Tyr182), and anti-MAPK p38 polyclonal antibody (all from Cell Signaling Technology Inc.).
Cells were seeded onto 12-well plates (3×105 cells/well), incubated for 5-10 min at room temperature, and then transfected with 5-25 nM CXCR4 siRNA or CXCR7 siRNA (Qiagen, Hilden, Germany) by using the HiperFect transfection reagent (Qiagen), according to the manufacturer's instructions. Cells were cultured in normal growth media for 24-72 hr after the transfection. Transfection efficiency of control siRNA was evaluated by fluorescence microscopy. Suppression of the cell surface levels of CXCR4 and CXCR7 was analyzed by flow cytometry.
All the leukemia cells and primary CD34+ leukemia cells expressed CXCR4 on their cell surface (data not shown). SDF-1 induced the transmigration of leukemia cells into the lower chamber of a Transwell system, which was abolished by treating the cells in the upper chamber with AMD3100 and T140 (Fig. 1A). Pretreating the cells in the upper chamber with PTX (200 ng/mL) for 2 hr also markedly inhibited the chemotaxis of the cells in response to SDF-1 (data not shown). To further understand the activities of AMD3100 and T140, we examined whether these agents induce the internalization of cell surface CXCR4. Treatment of U937 cells with 10-5 M AMD3100 or 10-6 M T140 for 3 hr in serum-free X-VIVO medium resulted in substantial internalization of surface CXCR4 (Fig. 1B).
SDF-1 alone did not affect the proliferation of the leukemia cells (data not shown). AMD3100, but not T140, increased the number of the leukemia cells, in a dose-dependent manner (Fig. 2A, B). After 3-day incubation, 10-5 M AMD3100 increased the proliferation of MO7e, HL-60, KG1a, K562, and U937 cells by 1.3-, 1.2-, 1.5-, 1.6-, and 1.6-fold, respectively, compared with the control (all P<0.05). Similar results were observed for primary CD34+ cells (Fig. 2C). Pretreating the cells with PTX for 2 hr did not abrogate the increase in proliferation (Fig. 2D). Additionally, AMD3100 recruited more leukemia cells into the S phase (Fig. 2E).
To investigate whether AMD3100 affected the apoptosis of leukemia cells, U937 cells were incubated in RPMI-1640 medium in the absence FBS. After 72 hr, 16.9%±3.4% of the cells tested positive for annexin V. The addition of AMD3100 slightly reduced this to 9.5%±2.9% (P<0.05; Fig. 2F). Similar results were obtained for other leukemia cells (data not shown).
We examined whether AMD3100 induced the phosphorylation of Stat3, MAPK p38, Akt, and MAPK p44/p42, which are involved in SDF-1-mediated signaling, by using MO7e cells. Stat3, MAPK p38, Akt, and MAPK p44/p42 were constitutively phosphorylated in MO7e cells at various levels. AMD3100 (up to 10-5 M) induced modest phosphorylation of MAPK p44/p42, but not the others (Fig. 3A). Similar results were observed from other leukemia cells (data not shown). To further define the involvement of signaling pathway(s) in the AMD3100-induced proliferation of leukemia cells, a proliferation assay was performed in the presence of various signal-blocking agents. U937 cells were incubated for 3 days with signal-blocking agents (LY294002, wortmannin, PD98059, AG490, rapamycin, and SB203580) in the presence or absence of AMD3100. The phosphatidyl inositol-3 kinase inhibitor wortmannin, the MAPK p44/p42 inhibitor PD98059, the MAPK P38 inhibitor SB203580, and the mTOR inhibitor rapamycin did not affect AMD3100-induced or spontaneous proliferation of these cells (Fig. 3B). Similar results were observed with other leukemia cells (data not shown).
On the basis of a recent report that indicated that AMD3100 induced a marked inhibition of survival in leukemia cells cultured for extended periods of up to 14 days [19], we further evaluated the effects of AMD3100 on the proliferation of leukemia cells in extended culture conditions (up to 14 days). Control cells, which were incubated in serum-free medium alone, increased in total number for the first 5 days and decreased thereafter. A high concentration of AMD3100 (10-5 M) significantly enhanced the proliferation of leukemia cells during the first 5 days of incubation. In contrast, compared to the controls, AMD3100-treated cells exhibited a marked decrease in the cell number after 5-7 days of incubation (Fig. 4A). Addition of SDF-1 at the beginning and at the middle of the incubation period did not affect the early increase or late decrease in the cell number (data not shown). In tumor cell colony assays using K562 cells, the number of colonies increased in the presence of AMD3100 during the early period of the assay, but markedly decreased in the later period of the assay (Fig. 4B).
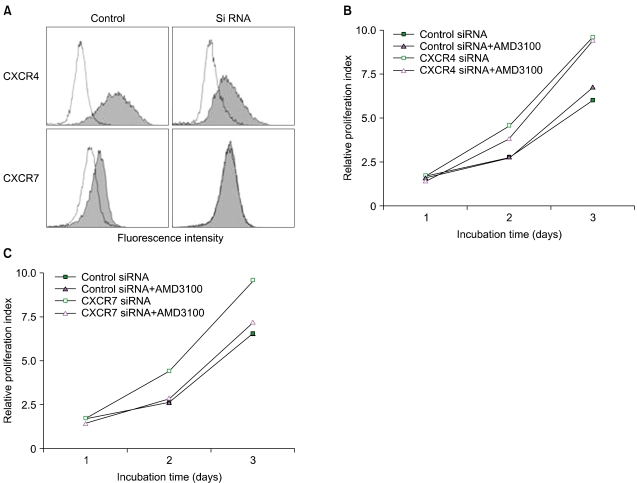
Recently, AMD3100 was shown to bind to CXCR7 and to act as an allosteric agonist of this receptor [20], suggesting that CXCR7 may be involved in AMD3100-induced proliferation enhancement. CXCR7/RDC-1 has been recently reported as another receptor for SDF-1 in many tumor cell lines [21]. Thus, we examined whether the cells expressed CXCR7/RDC-1 on their surface. CXCR7/RDC-1 was expressed at various levels in all the cells examined and was partially internalized after treatment with AMD3100, but not with T140 (Fig. 5). We next examined whether the knockdown of CXCR7 affected the aforementioned proliferation enhancement. Transfection of CXCR4 siRNA into U937 cells suppressed the expression of cell surface CXCR4, but it did not alter the proliferation enhancement. In contrast, the knockdown of CXCR7 significantly delayed the early proliferation enhancement (Fig. 6).
AMD3100, a specific antagonist of CXCR4, blocks the binding of SDF-1 to CXCR4, resulting in the inhibition of the SDF-1-induced migration or proliferation of many cell types [19, 22]. However, few studies have addressed whether AMD3100 influences the survival or proliferation of myeloid leukemia cells [19, 22, 23]. Since myeloid leukemia cells produce SDF-1 [24], which possibly acts as an autocrine growth factor, AMD3100 was expected to inhibit the proliferation of these cells. Indeed, the CXCR4 antagonist 4F-benzoyl-TN14003 has been shown to inhibit leukemia tumor growth [23]. We report here that AMD3100, but not T140, at a high concentration, for example 10-5 M, stimulates the proliferation of myeloid leukemia cells during short-term incubation (up to 5 days) and protects cells from serum deprivation-induced apoptosis.
We found that AMD3100 induced the phosphorylation of SDF-1-linked signaling molecules in leukemia cells. However, the proliferation-enhancing effects of AMD3100 in leukemia cells were not abrogated by pretreating the cells with PTX, a strong G protein-coupled receptor antagonist. Since CXCR4 is a PTX-sensitive G protein-coupled receptor [25], these results indicate that AMD3100 acts via pathways other than that mediated by CXCR4, to enhance cell proliferation. In our previous study, we have shown that AMD3100 enhanced the survival and proliferation of myeloma cells; the action of AMD3100 is not limited to a single cell type; and the proliferation enhancement induced by AMD3100 was markedly inhibited by PTX [18]. The reasons of the differences between myeloma and leukemia cells regarding the effects of PTX remain unclear and require further study.
Recently, CXCR7 has been identified as an alternative receptor for SDF-1 [26], and it has been shown to be involved in the endothelial adhesion and proliferation of several normal and malignant cell types, rather than their migration [27,28]. We have shown that AMD3100 induces the internalization of not only CXCR4, but also CXCR7, indicating that AMD3100 interacts with CXCR7, consistent with a previous report demonstrating that AMD3100 binds to CXCR7 and acts as an allosteric agonist [20]. G protein-coupled receptors consist of 7-transmembrane domain receptors, including chemokine receptors, which signal through heterodimeric G proteins. It has been demonstrated that ligand binding to CXCR7 does not activate typical G-protein signaling pathways, but activates MAP kinases through beta-arrestin [29]. We found that AMD3100 phosphorylated MAPK p44/p42 and that knockdown of CXCR7, but not CXCR4, delayed the initial proliferation enhancement. Altogether, it is suggested that the early proliferation enhancement induced by AMD3100 is exerted via CXCR7, and not CXCR4.
AMD3100 was shown to inhibit the outgrowth of leukemia colony-forming units in vitro [22]. AMD3100 at a concentration of 10-5 M has also been shown to strongly impair the survival and stimulate the differentiation of leukemia cells, such as HL60 and U937, cultured for 7-9 days [19]. However, these results appear contradictory. In the present study, 10-5 M AMD3100 enhanced the proliferation of the cells during the early course of incubation. After 5-7 days of culture, compared to the control cells, the number of cells treated with AMD3100 decreased more rapidly. Thus, the results from the 2 studies did not actually differ. It remains unclear whether the decrease in the cell number and increase in the cell apoptosis were induced directly by AMD3100, because higher cell density due to the increase in the cell number induced by AMD3100 in the early period of incubation might deteriorate the culture condition. Conversely, the rapid cell death later in long-term incubation in vitro may be attributed to the AMD3100-mediated SDF-1/CXCR4 blockade. Normal hematopoietic cells produce SDF-1, which forms an autocrine/paracrine regulatory loop [30]. Because leukemia cells produce SDF-1 [24], it is possible that AMD3100 binding to CXCR4 blocks the autocrine loop of SDF-1. However, the mechanisms involved in AMD3100-induced cell death need to be further elucidated.
In summary, the effects of CXCR4 antagonists on the proliferation of myeloid leukemia cells are not uniform. AMD3100, but not T140, enhances the survival and proliferation of myeloid leukemia cells during the early period of incubation in vitro. Although the observed proliferation enhancement was modest and only apparent in the early period of incubation and at a high AMD3100 concentration, caution must be exercised when considering the clinical application of this drug in patients with myeloid leukemia. Conversely, the inhibition of the proliferation and enhancement of apoptosis during the later period of incubation suggest a potential therapeutic use for AMD3100 in myeloid leukemia.
References
1. Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848. PMID: 9933168.

2. Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998; 91:4523–4530. PMID: 9616148.
3. Durig J, Rosenthal C, Elmaagacli A, et al. Biological effects of stroma-derived factor-1 alpha on normal and CML CD34+ haemopoietic cells. Leukemia. 2000; 14:1652–1660. PMID: 10995013.
4. Voermans C, van Heese WP, de Jong I, Gerritsen WR, van Der Schoot CE. Migratory behavior of leukemic cells from acute myeloid leukemia patients. Leukemia. 2002; 16:650–657. PMID: 11960346.

5. Majka M, Rozmyslowicz T, Honczarenko M, et al. Biological significance of the expression of HIV-related chemokine coreceptors (CCR5 and CXCR4) and their ligands by human hematopoietic cell lines. Leukemia. 2000; 14:1821–1832. PMID: 11021758.

6. Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004; 104:550–557. PMID: 15054042.

7. Tavor S, Petit I, Porozov S, et al. Motility, proliferation, and egress to the circulation of human AML cells are elastase dependent in NOD/SCID chimeric mice. Blood. 2005; 106:2120–2127. PMID: 15941909.

8. Dommange F, Cartron G, Espanel C, et al. CXCL12 polymorphism and malignant cell dissemination/tissue infiltration in acute myeloid leukemia. FASEB J. 2006; 20:1913–1915. PMID: 16818471.
9. Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006; 12:657–664. PMID: 16715089.

10. Peled A, Hardan I, Trakhtenbrot L, et al. Immature leukemic CD34+ CXCR4+ cells from CML patients have lower integrin-dependent migration and adhesion in response to the chemokine SDF-1. Stem Cells. 2002; 20:259–266. PMID: 12004084.
11. Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009; 23:43–52. PMID: 18987663.

12. Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998; 4:72–77. PMID: 9427609.

13. Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006; 107:3772–3778. PMID: 16439684.

14. Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009; 113:6206–6214. PMID: 19050309.

15. Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009; 113:6215–6224. PMID: 18955566.

16. Zhang WB, Navenot JM, Haribabu B, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 2002; 277:24515–24521. PMID: 11923301.

17. Watanabe M, Matsuyama W, Shirahama Y, et al. Dual effect of AMD3100, a CXCR4 antagonist, on bleomycin-induced lung inflammation. J Immunol. 2007; 178:5888–5898. PMID: 17442973.

18. Kim HY, Hwang JY, Kim SW, et al. The CXCR4 antagonist AMD3100 has dual effects on survival and proliferation of myeloma cells in vitro. Cancer Res Treat. 2010; 42:225–234. PMID: 21253325.
19. Tavor S, Eisenbach M, Jacob-Hirsch J, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008; 22:2151–5158. PMID: 18769446.

20. Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009; 75:1240–1247. PMID: 19255243.

21. Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006; 203:2201–2213. PMID: 16940167.

22. Liesveld JL, Bechelli J, Rosell K, et al. Effects of AMD3100 on transmigration and survival of acute myelogenous leukemia cells. Leuk Res. 2007; 31:1553–1563. PMID: 17403536.

23. Beider K, Begin M, Abraham M, et al. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol. 2011; 39:282–292. PMID: 21138752.

24. Tavor S, Petit I, Porozov S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004; 64:2817–2824. PMID: 15087398.

25. Moepps B, Frodl R, Rodewald HR, Baggiolini M, Gierschik P. Two murine homologues of the human chemokine receptor CXCR4 mediating stromal cell-derived factor 1alpha activation of Gi2 are differentially expressed in vivo. Eur J Immunol. 1997; 27:2102–2112. PMID: 9295051.
26. Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005; 280:35760–35766. PMID: 16107333.

27. Miao Z, Luker KE, Summers BC, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007; 104:15735–15740. PMID: 17898181.

28. Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br J Cancer. 2008; 99:1493–1501. PMID: 18854833.

29. Rajagopal S, Kim J, Ahn S, et al. Beta-arrestin- but not G protein-mediated signaling by "the decoy" receptor CXCR7. Proc Natl Acad Sci U S A. 2010; 107:628–632. PMID: 20018651.
30. Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002; 99:792–799. PMID: 11806978.

Fig. 1
AMD3100 and T140 inhibit the SDF-1-induced chemotaxis of myeloid leukemia cells, and trigger the internalization of surface CXCR4. (A) Four-hour transmigration of MO7e cells towards SDF-1 (200 ng/mL). AMD3100 and T140 were added at concentrations of 10-5 M and 10-6 M, respectively. a)P<0.05, compared to the control. (B) U937 cells were incubated with or without 10-5 M AMD3100 and 10-6 M T140, respectively, for 3 hr and subsequently subjected to flow cytometry.
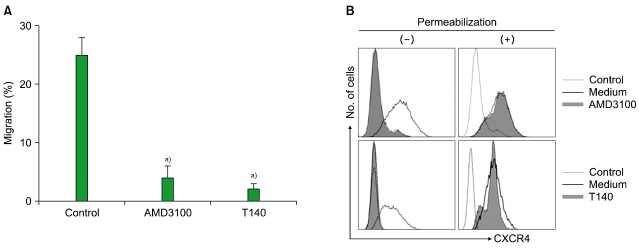
Fig. 2
AMD3100 stimulates the proliferation of myeloid leukemia cells. Cells were incubated in the presence of AMD3100 (A) or T140 (B) for up to 72 hr. (C) Bone marrow CD34+ cells from 3 each patients with AML and CML were incubated in the presence of AMD3100 (10-5 M) for 72 hr. (D) U937 cells were incubated with AMD3100 (10-5 M). As indicated, the cells were pretreated with pertussis toxin (PTX) for 2 hr before the incubation. (E) U937 cells were incubated in X-VIVO medium with or without AMD3100 (10-5 M) and subsequently subjected to cell cycle analysis. (F) U937 cells were incubated in RPMI-1640 medium without serum in the presence or absence of AMD3100 (10-5 M) for 72 hr, stained with annexin V and propidium iodide (PI), and subjected to flow cytometry. Representative flow cytometry profiles are shown in the left panel. In the right panel, the percentages of the annexin V-positive apoptotic cells are presented a)P<0.05, compared with the control.
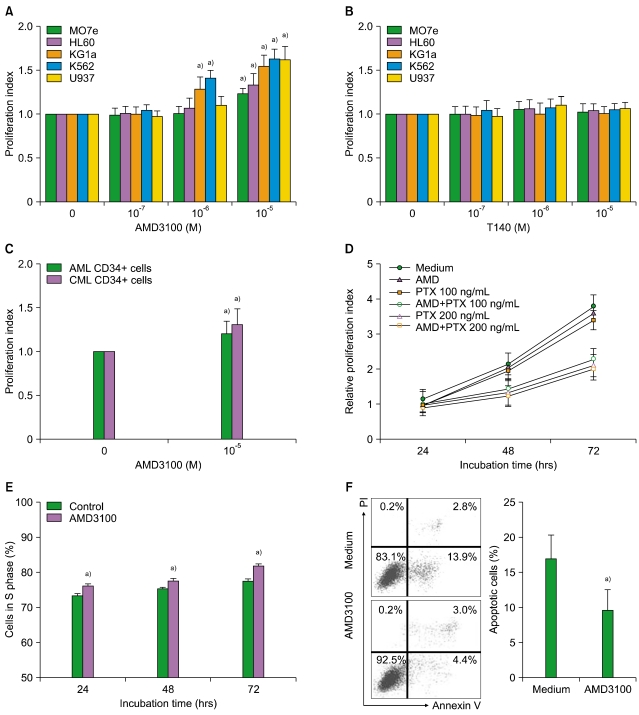
Fig. 3
AMD3100 induces the phosphorylation of SDF-1-linked signaling molecules. (A) MO7e cells were incubated with AMD3100 (10-5 M) and subsequently subjected to western blot analysis. Representative results of 3 experiments are shown. (B) U937 cells were treated with 10-5 M AMD3100, 10 µM LY294002, 100 nM wortmannin, 50 µM PD98059, 10 µM AG490, 50 nM rapamycin, and 10 µM SB203580 for 3 days.
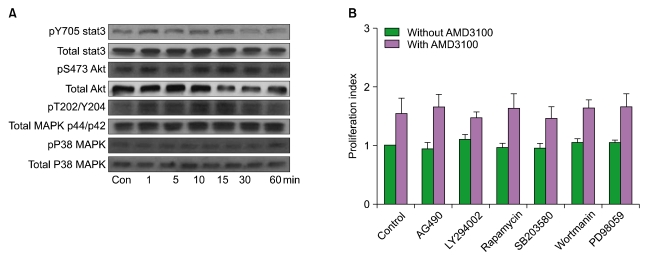
Fig. 4
Survival of leukemia cells in an extended period of culture. (A) HL-60 cells (a), K562 cells (b), KG1a cells (c), and U937 cells (d) were incubated with AMD3100 (10-7 M to 10-5 M) for up to 14 days. Data are representative results of 3 or more independent experiments showing similar results. (B) Effects of AMD3100 on the formation of tumor cell colonies were examined using K562 cells. Representative profiles of the colony assay are shown.
Fig. 5
Myeloid leukemia cells express CXCR7 on the cell surface, which is internalized by AMD3100. (A) Representative profiles of CXCR7 expression on the cell surface. (B) U937 cells were incubated with 10-5 M AMD3100 and 10-5 M T140, respectively, for 3 hr and subsequently subjected to flow cytometry.
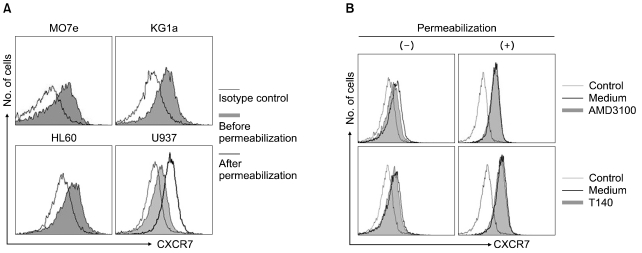
Fig. 6
Knockdown of CXCR7, but not CXCR4, delays the proliferation enhancement induced by AMD3100. U937 cells were transfected with 25 nM CXCR4 siRNA or 5 nM CXCR7 siRNA and were further incubated in serum-free medium in the presence or absence of AMD3100 for up to 3 days. (A) Representative profiles of the decrease in cell surface expression of CXCR4 and CXCR7 induced by siRNA. (B) Representative results of proliferation of the CXCR4 siRNA-transfected cells among 3 or more independent experiments showing similar results. (C) Representative results of the proliferation of the CXCR7 siRNA-transfected cells among 3 or more independent experiments showing similar results.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download