1. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008; 25:291–295. PMID:
18577030.

2. Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008; 50:304–307. PMID:
17455317.

3. Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008; 20:23–29. PMID:
18197035.

4. Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001; 138:728–734. PMID:
11343051.

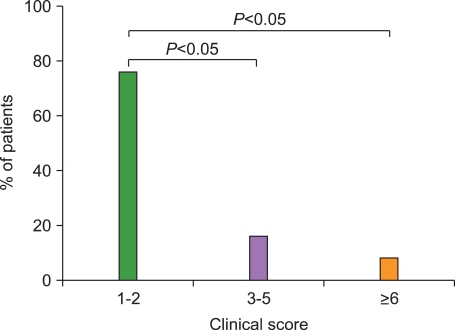
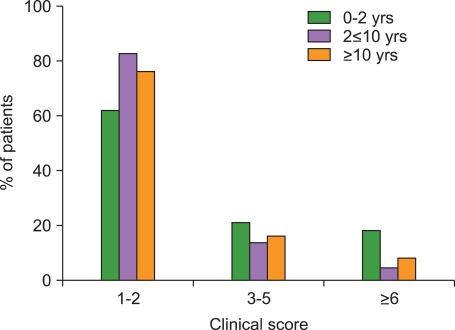
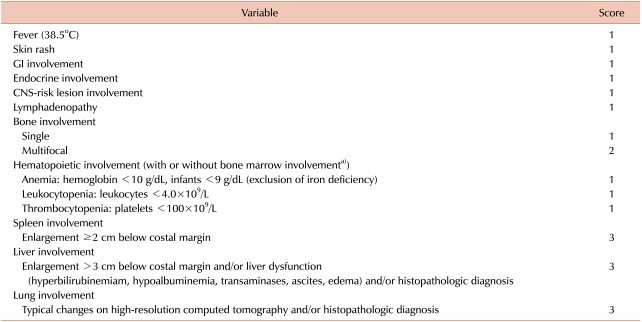
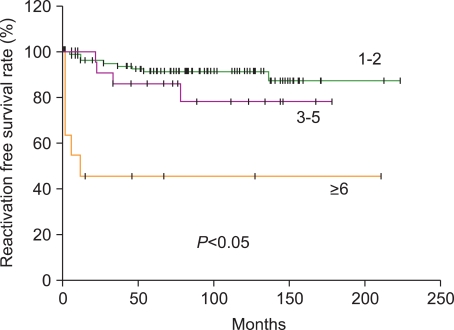
5. Donadieu J, Piguet C, Bernard F, et al. A new clinical score for disease activity in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2004; 43:770–776. PMID:
15390280.

6. Jubran RF, Marachelian A, Dorey F, Malogolowkin M. Predictors of outcome in children with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2005; 45:37–42. PMID:
15768381.

7. Glotzbecker MP, Carpentieri DF, Dormans JP. Langerhans cell histiocytosis: clinical presentation, pathogenesis, and treatment from the LCH etiology research group at the Children's Hospital of Philadelphia. UPOJ. 2002; 15:67–73.
8. Kilpatrick SE, Wenger DE, Gilchrist GS, Shives TC, Wollan PC, Unni KK. Langerhans' cell histiocytosis (histiocytosis X) of bone. A clinicopathologic analysis of 263 pediatric and adult cases. Cancer. 1995; 76:2471–2484. PMID:
8625073.

9. Alston RD, Tatevossian RG, McNally RJ, Kelsey A, Birch JM, Eden TO. Incidence and survival of childhood Langerhans' cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer. 2007; 48:555–560. PMID:
16652350.

10. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008; 25:291–295. PMID:
18577030.

11. Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008; 20:23–29. PMID:
18197035.

12. Bechan GI, Egeler RM, Arceci RJ. Biology of Langerhans cells and Langerhans cell histiocytosis. Int Rev Cytol. 2006; 254:1–43. PMID:
17147996.

13. Chikwava KR, Hunt JL, Mantha GS, Murphy JE, Jaffe R. Analysis of loss of heterozygosity in single-system and multisystem Langerhans' cell histiocytosis. Pediatr Dev Pathol. 2007; 10:18–24. PMID:
17378622.

14. Betts DR, Leibundgut KE, Feldges A, Plüss HJ, Niggli FK. Cytogenetic abnormalities in Langerhans cell histiocytosis. Br J Cancer. 1998; 77:552–555. PMID:
9484810.

15. Scappaticci S, Danesino C, Rossi E, et al. Cytogenetic abnormalities in PHA-stimulated lymphocytes from patients with Langerhans cell histocytosis. AIEOP-Istiocitosi Group. Br J Haematol. 2000; 111:258–262. PMID:
11091209.
16. Schouten B, Egeler RM, Leenen PJ, Taminiau AH, van den Broek LJ, Hogendoorn PC. Expression of cell cycle-related gene products in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002; 24:727–732. PMID:
12468913.

17. Bank MI, Rengtved P, Carstensen H, Petersen BL. p53 expression in biopsies from children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002; 24:733–736. PMID:
12468914.

18. Petersen BL, Rengtved P, Bank MI, Carstensen H. High expression of markers of apoptosis in Langerhans' cell histiocytosis. Histopathology. 2003; 42:186–193. PMID:
12558751.

19. Weintraub M, Bhatia KG, Chandra RS, Magrath IT, Ladisch S. p53 expression in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 1998; 20:12–17. PMID:
9482407.

20. Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E. Differential in situ cytokine profiles of Langerhans'-like cells and T cells in Langerhans' cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999; 94:4195–4201. PMID:
10590064.
21. Annels NE, Da Costa CE, Prins FA, Willemze A, Hogendoorn PC, Egeler RM. Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis. J Exp Med. 2003; 197:1385–1390. PMID:
12743170.

22. Andersson By U, Tani E, Andersson U, Henter JI. Tumor necrosis factor, interleukin 11, and leukemia inhibitory factor produced by Langerhans cells in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2004; 26:706–711. PMID:
15543003.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download