Abstract
Background
BAFF (B cell-activating factor) and APRIL (a proliferation-inducing ligand) are members of the tumor necrosis factor family and promote B cell survival and proliferation. We evaluated the correlation between serum concentration of BAFF or APRIL and severity of acute graft-versus-host disease (GVHD).
Methods
Fifteen patients who received allogeneic hematopoietic stem transplantation for leukemia and developed acute GVHD were enrolled. We determined serum concentrations of BAFF and APRIL at the onset of the first clinical manifestation of GVHD by enzyme-linked immunosorbent assay.
Results
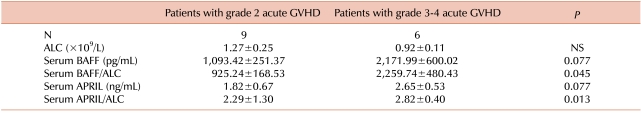
Nine patients had grade 2 acute GVHD, and 6 had grade 3-4 acute GVHD. The BAFF serum concentration was higher in patients with grade 3-4 acute GVHD (1,093.42 in grade 2 vs. 2,171.99 pg/mL in grade 3-4), although the difference was not significant (P=0.077). However, the ratio of BAFF serum concentration to absolute lymphocyte count (ALC) (BAFF/ALC) was significantly higher in patients with grade 3-4 acute GVHD (P=0.045). The APRIL serum concentration and APRIL/ALC ratio showed similar results (P=0.077 and P=0.013, respectively).
Conclusion
Patients with grade 3-4 acute GVHD had higher BAFF/ALC and APRIL/ALC ratios than patients with grade 2 acute GVHD. These findings suggest that B cells might play an important role in the development of acute GVHD, and that the BAFF and APRIL concentrations in serum might be significant predictive factors for estimating the severity of acute GVHD. Their clinical significance should be further evaluated in a larger patient population.
Graft-versus-host disease (GVHD) is a significant cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT), and is a major barrier against successful allogeneic HSCT [1]. GVHD is a complex disease resulting from an exaggerated immune response of the donor's CD4+ and CD8+ T cells against the recipient's tissue. In acute GVHD, the subsequent proliferation and differentiation of these activated T cells stimulates additional effectors that mediate tissue damage, including cytotoxic T cells, natural killer (NK) cells, tumor necrosis factor (TNF)-α, and IL-1. Although the pathophysiology of chronic GVHD remains poorly understood, T cells have been identified as key players in both acute and chronic GVHDs, and most established drugs for GVHD have been developed to target T cells [2]. However, few effective therapies are currently available for the treatment of GVHD, and the currently used immunosuppressive agents are mostly nonspecific and, unfortunately, are associated with severe adverse effects, including serious infections [2]. Therefore, a better understanding of the basic mechanisms underlying the development of GVHD is urgently needed.
Recently, several studies have demonstrated that B cells are also involved in the immunopathophysiology of acute and chronic GVHDs [3-5]. Early-phase clinical trials indicate that B cell depletion with rituximab has beneficial effects on both acute and chronic GVHDs [3].
BAFF (B cell-activating factor) and APRIL (a proliferation-inducing ligand) are members of the TNF family that act as B cell trophic factors, promoting the survival and proliferation of B cells [6]. Both BAFF and APRIL and their receptors are strongly up-regulated in many autoimmune disorders [3, 6, 7]. Because the clinical features of GVHD are similar to those of autoimmune diseases, the elevated serum concentrations of BAFF and APRIL seen in GVHD may be closely related to the processes underlying its development. Several evidences clearly demonstrate that B cells are involved in the pathogenesis of chronic GVHD. Furthermore, the highest BAFF concentrations were found in the sera of patients with chronic GVHD, and these were significantly higher than in patients with inactive disease or in patients who never developed chronic GVHD after allogeneic HSCT [8, 9]. A recent study suggested that high BAFF concentration during the peri-transplantation period may be predictive of acute GVHD [10]. However, whether B cells are involved in acute GVHD is still controversial.
Higher grades of acute GVHD are often unresponsive to high doses of steroid therapy and, as a result, frequently have fatal outcomes [1, 2]. Therefore, early diagnosis and prediction of the severity of acute GVHD may be very important. However, acute GVHD is frequently diagnosed only by close examination of skin lesions or monitoring of clinical signs such as diarrhea, and only a few biochemical tests are available to accurately diagnose and predict the severity of GVHD [11].
There have been only a few reports regarding the relationship between elevated serum BAFF and the development of acute GVHD. In this study, we assessed the value of serum BAFF and APRIL measurements taken at diagnosis of acute GVHD in estimating the severity of the condition.
Fifteen patients who received allogeneic HSCT for leukemia and developed acute GVHD were enrolled in this study. All patients received GVHD prophylaxis with a calcineurin inhibitor (cyclosporine or tacrolimus) with or without a short course of methotrexate (MTX), and none of the grafts were depleted of T cells. Acute GVHD was graded based on the revised system developed by the International Bone Marrow Transplant Registry (IBMTR) in 1997 [12]. In the absence of histologic or clinical signs or symptoms of chronic GVHD, the persistence, recurrence, or new onset of characteristic skin, gastrointestinal tract, or liver abnormalities were classified as acute GVHD regardless of the time after transplantation.
All blood samples were collected at the onset of the first clinical manifestation of GVHD and diagnosis of acute GVHD. Serum was separated from whole blood cells via 10 minutes of centrifugation at 2,500 rpm. The serum samples were stored at -80℃ until tested for levels of BAFF and APRIL.
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used to measure the concentrations of BAFF and APRIL. The concentration of serum BAFF was measured using the frozen serum samples after thawing. Fifty microliters of each serum was added to each well of an ELISA plate that was coated with a mouse monoclonal antibody against human BAFF (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's recommendations. After incubation at room temperature for 2 hrs, samples were aspirated and the plates were washed. The plates were then incubated for 2 hrs with an antibody against BAFF conjugated to horseradish peroxidase (HRP). Tetramethylbenzidine (TMB) was used as a substrate, and optical density was read at 450 nm. The serum concentration of BAFF (pg/mL) was calculated based on the standard curve produced with 40,000 pg/mL of recombinant human BAFF. The minimal detectable concentration of BAFF was 1.5 pg/mL. Each sample was tested in duplicate, and the average value was reported.
Serum levels of APRIL were also measured by ELISA (Human APRIL ELISA BMS2008; Bender MedSystems GmbH, Vienna, Austria). APRIL ELISA was performed according to the manufacturer's instructions. The color reaction and absorbance readings were performed in the same manner as for the BAFF ELISA. The serum concentration of APRIL (ng/mL) was calculated based on a standard curve from 50 ng/mL to 0 ng/mL, and the minimal detectable concentration of APRIL was 0.4 ng/mL. All samples were analyzed in duplicate, and the average value was reported.
The relationships between serum BAFF and APRIL levels and continuous clinical variables were evaluated using Spearman's correlation test. Mann-Whitney U-test was used to evaluate the relationship of serum BAFF and APRIL levels with severity of acute GVHD. All statistical analyses were conducted using SPSS 18.0 (SPSS Inc, Chicago, IL, USA) and a 2-sided P-value of less than 0.05 was considered statistically significant.
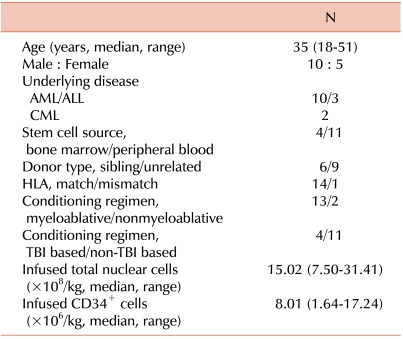
Clinical characteristics of the 15 enrolled patients are described in Table 1. The median patient age was 35 years (range, 18-51 years). This study included 9 patients with acute myelogenous leukemia (AML), 4 with acute lymphoblastic leukemia (ALL), and 2 with chronic myelogenous leukemia (CML). Fourteen patients received HLA-identical allogeneic HSCT (6 related and 8 unrelated) and 1 patient received HLA-mismatched unrelated allogeneic HSCT. All patients received non-T cell-depleted grafts. A myeloablative conditioning regimen was used in all but 2 cases. Total body irradiation (TBI) was used as the conditioning regimen for 4 patients (1,320 cGy of TBI and cyclophosphamide).
Ten patients received cyclosporine-based GVHD prophylaxis (3 mg/kg/day adjusted for blood levels between 200 and 400 ng/mL): 4 received cyclosporine alone and 6 received cyclosporine and a short course of MTX. MTX was administered at a dosage of 10 mg/m2 on days +1, +3, +6, and +11. Tacrolimus-based prophylaxis was administered to 5 patients who received unrelated HSCT: 4 received tacrolimus alone and 1 received tacrolimus and a short course of MTX. Tacrolimus was administered intravenously as a continuous infusion at a dose of 0.03 mg/kg from days -1 to +21, and then orally at a total dose of 0.12 mg/kg/day, divided into 2 doses, to maintain a blood level of 10-20 ng/mL. For the patients with grade 2 or higher GVHD, intravenous methylprednisolone (2 mg/kg/day) was administered.
We categorized the patients into 2 groups according to the severity of acute GVHD: patients presenting with grade 2 acute GVHD (N=9) and patients with grade 3-4 acute GVHD (N=6).
Patients' clinical characteristics and the serum concentrations of BAFF and APRIL are summarized in Table 2. The mean serum concentration of BAFF at diagnosis of acute GVHD was 1,524.85±1,181.34 pg/mL (mean±standard deviation). The median concentration of BAFF was 966.43 pg/mL. The mean serum BAFF concentration of the control patients (4 patients without GVHD) was 1,107.84 pg/mL. The median absolute lymphocyte count (ALC) at diagnosis of acute GVHD was 0.92×109/L. The median serum concentration of BAFF to absolute lymphocyte count ratio (BAFF/ALC) was 1,073.81. The serum concentration of APRIL at diagnosis of acute GVHD was 2.15±1.76 ng/mL (median, 1.36 ng/mL). The mean serum APRIL concentration of the control patients was 0.98 ng/mL. The median serum concentration of APRIL to absolute lymphocyte count ratio (APRIL/ALC) was 1.42.
The patients who had higher BAFF levels showed a trend of elevated APRIL levels, although the difference was not significant (P=0.060, Spearman's correlation test). However, the value of BAFF/ALC showed a significant correlation with the value of APRIL/ALC (P=0.024, Spearman's correlation test). There was no significant correlation between BAFF/ALC or APRIL/ALC level and onset date of acute GVHD (P=0.859, 0.086, respectively).
The BAFF/ALC or APRIL/ALC ratios at the diagnosis of acute GVHD had no correlation with the degree of response to high-dose steroid therapy for acute GVHD or the risk of relapse after the initial high dose of steroids.
When we divided the enrolled patients into 2 groups according to the severity of acute GVHD, 9 patients had grade 2 acute GVHD and 6 patients suffered from grade 3-4 acute GVHD (Table 3). The serum concentration of BAFF was higher in the patients with grade 3-4 acute GVHD (2,171.99±600.02 pg/mL) than in the patients with grade 2 acute GVHD (1,093.42±251.37 pg/mL), although the difference was not significant (P=0.077). However, the patients with grade 3-4 acute GVHD showed a significantly higher BAFF/ALC ratio (2,259.74±480.43) than the patients with grade 2 acute GVHD (925.24±168.53, P=0.045). The patients with grade 3-4 acute GVHD also had a trend for higher serum concentrations of APRIL (P=0.077) and a significantly higher APRIL/ALC ratio (2.29±1.30 in grade 2 vs. 2.82±0.40 in grade 3-4, P=0.013) (Table 3).
This study showed that BAFF/ALC or APRIL/ALC values at diagnosis of acute GVHD are associated with the severity of the condition. Therefore, B cells might play an important role in development and progression of acute GVHD. As a result, the BAFF/ALC or APRIL/ALC ratio at diagnosis of acute GVHD might be used as a predictive factor to estimate its final severity.
Although the role of B cells in the development of chronic GVHD is relatively well known, their role in the development of acute GVHD is still controversial. Furthermore, it is very difficult to evaluate the role of B cells from the donor or recipient in the development of acute GVHD, because the reconstitution of the B cell compartment required for humoral immunity may take up to 2 years after HSCT [13]. There is some evidence for adverse effects of donor B cells. High numbers of B cells in apheresis products correlated with an increased incidence of acute GVHD and increased treatment-related mortality [14]. Early-phase clinical trials indicate that B cell depletion with rituximab has beneficial effects on acute GVHD [3]. We showed that a higher serum concentration of B cell-activating factors such as BAFF or APRIL at diagnosis of acute GVHD might be associated with a higher grade of acute GVHD. Therefore, we concluded that B cells might play an important role in the development of acute GVHD, and BAFF/ALC or APRIL/ALC might be significant predictive factors for estimating the severity of the condition.
A higher grade of acute GVHD is often accompanied by unresponsiveness to high-dose steroid therapy and, frequently, a fatal outcome. Many researchers have looked for new biomarkers to diagnose and predict the severity of acute GVHD. Such a test could help physicians decide more quickly, which patients need urgent treatment. The diagnosis of acute GVHD is still performed solely by close examination of skin lesions or monitoring of clinical signs such as diarrhea, and it is very difficult to predict the final severity of acute GVHD at initial diagnosis using these methods. Several biomarkers associated with T cell activation such as soluble CD8, soluble IL-2 receptor, soluble CD40 ligand, and soluble CD28 have been evaluated for detecting higher grades of acute GVHD, before signs and symptoms emerge [11]. However, there have been no reports of biomarkers associated with B cell activation. Although our results suggest that a B cell-activating factor such as BAFF or APRIL might be an effective biomarker to predict the severity of acute GVHD, further study involving a larger number of patients should be performed to confirm the roles of these biomarkers in relation to B cell activation.
We evaluated the role of BAFF and APRIL at the onset of acute GVHD as predictive biomarkers for the development of acute GVHD. APRIL, like BAFF, is a molecule belonging to the TNF family, and is produced mainly by the same cells as BAFF [6, 7]. Because APRIL is a homologue of BAFF, and recent evidence has also identified it as an important survival factor in several human B-cell malignancies, such as chronic lymphocytic leukemia [15], we evaluated the predictive value of serum APRIL for acute GVHD in this study. Although a small number of reports have demonstrated a relationship between serum BAFF and acute GVHD [10], there have been no reports regarding changes of serum APRIL in patients with acute GVHD. According to our results, the value of APRIL/ALC showed a significant correlation with the value of BAFF/ALC (P=0.024, Spearman's correlation test). Therefore, APRIL might also be of value in predicting the severity of acute GVHD, and activation of B cells may have a significant role in the development of acute GVHD. Considering these possibilities, anti-CD20 monoclonal antibody (rituximab) as well as a human monoclonal antibody against BAFF (belimumab) or APRIL might be beneficial in the treatment of acute GVHD. These findings should be analyzed in further studies with a larger patient population.
In this study, we evaluated the BAFF/ALC and APRIL/ALC ratios as well as BAFF and APRIL serum concentrations. Because the amount of BAFF per B cell is a critical determinant of autoreactive B cell survival, and the BAFF/B cell ratio is significantly higher in patients with chronic GVHD compared with healthy individuals [9], we additionally analyzed BAFF and APRIL levels according to lymphocyte count in this study. Although absolute lymphocyte count does not exactly correlate with B cell count, we used the ALC in this study because it is easier to obtain in daily clinical practice. According to our results, BAFF/ALC or APRIL/ALC ratios might be better predictors of the severity of acute GVHD than BAFF or APRIL levels.
This study was the first report to analyze the relationship between the serum concentration of BAFF or APRIL at diagnosis of acute GVHD and the severity of the condition. Although BAFF and APRIL were not significant predictive markers in this study, our results suggest that they might still be important biomarkers for predicting the severity of acute GVHD.
In conclusion, we showed that patients with grade 3-4 acute GVHD had BAFF/ALC and APRIL/ALC values that were significantly higher than those of patients with grade 2 acute GVHD. B cell-related biomarkers such as BAFF or APRIL might be useful for evaluating acute GVHD severity, and B cell-targeted or BAFF/APRIL-targeted therapeutic approaches for the treatment of acute GVHD might give significant benefits. The clinical significance of this study should be further evaluated in a larger patient population.
References
1. Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001; 19:3685–3691. PMID: 11504750.

2. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009; 373:1550–1561. PMID: 19282026.

3. Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009; 114:4919–4927. PMID: 19749094.
4. Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009; 114:4327–4336. PMID: 19713461.

5. Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008; 93:1702–1711. PMID: 18728020.

7. Yeramilli VA, Knight KL. Requirement for BAFF and APRIL during B cell development in GALT. J Immunol. 2010; 184:5527–5536. PMID: 20400696.

8. Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007; 13:6107–6114. PMID: 17947475.

9. Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009; 113:3865–3874. PMID: 19168788.

10. Cho BS, Min CK, Kim HJ, et al. High levels of B cell activating factor during the peritransplantation period are associated with a reduced incidence of acute graft-versus-host disease following myeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010; 16:629–638. PMID: 19963070.

11. August KJ, Chiang KY, Bostick RM, et al. Biomarkers of immune activation to screen for severe, acute GVHD. Bone Marrow Transplant. 2011; 46:601–604. PMID: 20622904.

12. Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997; 97:855–864. PMID: 9217189.

13. Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010; 115:3861–3868. PMID: 20215642.

14. Iori AP, Torelli GF, De Propris MS, et al. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation. 2008; 85:386–390. PMID: 18322430.

15. Guadagnoli M, Kimberley FC, Phan U, et al. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood. 2011; 117:6856–6865. PMID: 21543761.

Table 2
Serum BAFF and APRIL levels and acute GVHD characteristics.
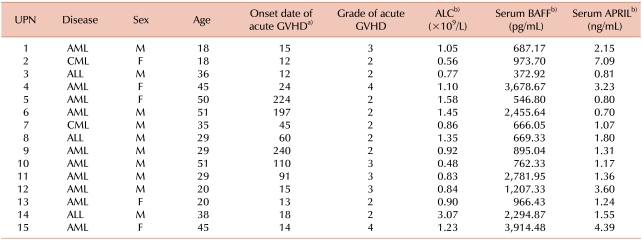
a)Onset date of acute GVHD after allogeneic HSCT, b)Laboratory results at the diagnosis of acute GVHD.
Abbreviations: AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; M, male; F, female; GVHD, graft-versus-host disease; ALC, Absolute lymphocyte count; BAFF, B cell-activating factor; APRIL, a proliferation-inducing ligand.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download