1. Cutler C, Antin JH. Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Manifestations and treatment of acute graft-versus-host disease. Thomas' hematopoietic cell transplantation. 2009. 4th ed. Oxford, UK: Wiley-Blackwell;p. 1287–1303.

2. Pavletic SZ, Vogelsang GB. Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Chronic graft-versus-host disease: clinical manifestations and therapy. Thomas' hematopoietic cell transplantation. 2009. 4th ed. Oxford, UK: Wiley-Blackwell;p. 1304–1324.

3. Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graftversus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69:204–217. PMID:
6996481.
4. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005; 11:945–956. PMID:
16338616.
5. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006; 12:31–47. PMID:
16399567.

6. Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006; 12:126–137. PMID:
16443511.

7. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2006; 12:252–266. PMID:
16503494.
8. Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006; 12:375–396. PMID:
16545722.

9. Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: VI. Design of Clinical Trials Working Group Report. Biol Blood Marrow Transplant. 2006; 12:491–505. PMID:
16635784.

10. Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004; 104:3501–3506. PMID:
15292060.

11. Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009; 114:702–708. PMID:
19470693.

12. Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011; 117:3214–3219. PMID:
21263156.

13. Arora M, Klein JP, Weisdorf DJ, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research Analysis. Blood. 2011; 117:6714–6720. PMID:
21493797.

14. Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009; 10:855–864. PMID:
19695955.

15. Mohty M, Labopin M, Balère ML, et al. Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Sociéte Française de Greffe de Moelle et de Thérapie Cellulaire. Leukemia. 2010; 24:1867–1874. PMID:
20882046.

16. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003; 9:215–233. PMID:
12720215.

17. Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981; 304:1529–1533. PMID:
7015133.
18. Sullivan KM, Weiden PL, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989; 73:1720–1728. PMID:
2653460.
19. Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990; 75:555–562. PMID:
2297567.

20. Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005; 23:1993–2003. PMID:
15774790.

21. Thepot S, Zhou J, Perrot A, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia. 2010; 24:1852–1858. PMID:
20827288.

22. Inamoto Y, Flowers ME, Lee SJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011; 118:456–463. PMID:
21633087.

23. Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988; 72:546–554. PMID:
3042041.

24. Koc S, Leisenring W, Flowers ME, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000; 96:3995–3996. PMID:
11090092.

25. Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001; 7:265–273. PMID:
11400948.

26. Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002; 100:48–51. PMID:
12070007.

27. Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graftversus-host disease. Blood. 2009; 113:5074–5082. PMID:
19270260.

28. Gilman AL, Schultz KR, Goldman FD, et al. Randomized trial of hydroxychloroquine for newly diagnosed chronic graftversus-host disease in children: a children's oncology group study. Biol Blood Marrow Transplant. 2011; [Epub ahead of print].

29. Martin PJ, Carpenter PA, Sanders JE, Flowers ME. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004; 79:221–228. PMID:
15168588.

30. Martin P, Gilman AL. Vogelsang GB, Pavletic SZ, editors. Front line treatment of chronic GVHD. Chronic graft versus host disease: interdisciplinary management. 2009. New York, NY: Cambridge University Press;p. 124–133.
31. Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002; 100:415–419. PMID:
12091330.

32. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009; 15:1143–1238. PMID:
19747629.

33. Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003; 101:407–414. PMID:
12393659.

34. Harter JG, Reddy WJ, Thorn GW. Studies on an intermittent corticosteroid dosage regimen. N Engl J Med. 1963; 269:591–596. PMID:
14044395.

35. Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010; 16:1611–1628. PMID:
20601036.

36. Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011; 17:1–17. PMID:
20685255.

37. Lee SJ, Vogelsang G, Gilman A, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002; 8:32–39. PMID:
11846354.

39. Mookerjee B, Altomonte V, Vogelsang G. Salvage therapy for refractory chronic graft-versus-host disease with mycophenolate mofetil and tacrolimus. Bone Marrow Transplant. 1999; 24:517–520. PMID:
10482936.

40. Busca A, Saroglia EM, Lanino E, et al. Mycophenolate mofetil (MMF) as therapy for refractory chronic GVHD (cGVHD) in children receiving bone marrow transplantation. Bone Marrow Transplant. 2000; 25:1067–1071. PMID:
10828867.

41. Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002; 30:287–295. PMID:
12209350.

42. Busca A, Locatelli F, Marmont F, Audisio E, Falda M. Response to mycophenolate mofetil therapy in refractory chronic graft-versus-host disease. Haematologica. 2003; 88:837–839. PMID:
12857569.
43. Kim JG, Sohn SK, Kim DH, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol. 2004; 73:56–61. PMID:
15182339.

44. Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005; 84:681–685. PMID:
16001244.

45. Lopez F, Parker P, Nademanee A, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005; 11:307–313. PMID:
15812396.

46. Takami A, Mochizuki K, Okumura H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol. 2006; 83:80–85. PMID:
16443558.

47. Onishi C, Ohashi K, Sawada T, et al. A high risk of life-threatening infectious complications in mycophenolate mofetil treatment for acute or chronic graft-versus-host disease. Int J Hematol. 2010; 91:464–470. PMID:
20217287.

48. Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009; 44:739–748. PMID:
19377515.

49. Vogelsang GB, Farmer ER, Hess AD, et al. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992; 326:1055–1058. PMID:
1549151.

50. Parker PM, Chao N, Nademanee A, et al. Thalidomide as salvage therapy for chronic graft-versus-host disease. Blood. 1995; 86:3604–3609. PMID:
7579470.

51. Rovelli A, Arrigo C, Nesi F, et al. The role of thalidomide in the treatment of refractory chronic graft-versus-host disease following bone marrow transplantation in children. Bone Marrow Transplant. 1998; 21:577–581. PMID:
9543061.

52. Browne PV, Weisdorf DJ, DeFor T, et al. Response to thalidomide therapy in refractory chronic graft-versus-host disease. Bone Marrow Transplant. 2000; 26:865–869. PMID:
11081386.

53. van de Poel MH, Pasman PC, Schouten HC. The use of thalidomide in chronic refractory graft versus host disease. Neth J Med. 2001; 59:45–49. PMID:
11476911.

54. Kulkarni S, Powles R, Sirohi B, et al. Thalidomide after allogeneic haematopoietic stem cell transplantation: activity in chronic but not in acute graft-versus-host disease. Bone Marrow Transplant. 2003; 32:165–170. PMID:
12838281.

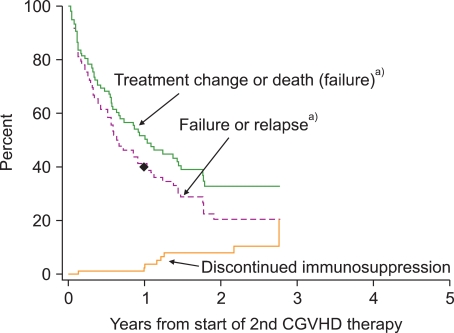
55. Flowers ME, Storer B, Carpenter P, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008; 14:1380–1384. PMID:
19041060.

56. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328:1490. PMID:
15205295.

57. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117:4190–4207. PMID:
21325604.

58. Cutler C, Miklos D, Kim HT, et al. Rituximab for steroidrefractory chronic graft-versus-host disease. Blood. 2006; 108:756–762. PMID:
16551963.

59. Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab. Bone Marrow Transplant. 2007; 40:273–277. PMID:
17549053.
60. von Bonin M, Oelschlägel U, Radke J, et al. Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation. 2008; 86:875–879. PMID:
18813113.

61. Kim SJ, Lee JW, Jung CW, et al. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study. Haematologica. 2010; 95:1935–1942. PMID:
20663943.

62. Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009; 114:719–722. PMID:
19289852.

63. Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009; 114:709–718. PMID:
19403889.

64. Jacobsohn DA, Chen AR, Zahurak M, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007; 25:4255–4261. PMID:
17878478.

65. Jacobsohn DA, Gilman AL, Rademaker A, et al. Evaluation of pentostatin in corticosteroid-refractory chronic graft-versus-host disease in children: a Pediatric Blood and Marrow Transplant Consortium Study. Blood. 2009; 114:4354–4360. PMID:
19745067.

66. Pidala J, Kim J, Roman-Diaz J, et al. Pentostatin as rescue therapy for glucocorticoid-refractory acute and chronic graft-versus-host disease. Ann Transplant. 2010; 15:21–29. PMID:
21183872.
67. Weng JY, Du X, Geng SX, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010; 45:1732–1740. PMID:
20818445.

68. Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998; 92:3098–3104. PMID:
9787144.

69. Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998; 4:27–37. PMID:
9701389.

70. Child FJ, Ratnavel R, Watkins P, et al. Extracorporeal photopheresis (ECP) in the treatment of chronic graft-versus-host disease (GVHD). Bone Marrow Transplant. 1999; 23:881–887. PMID:
10338042.

71. Alcindor T, Gorgun G, Miller KB, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001; 98:1622–1625. PMID:
11520818.

72. Salvaneschi L, Perotti C, Zecca M, et al. Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfusion. 2001; 41:1299–1305. PMID:
11606832.
73. Apisarnthanarax N, Donato M, Körbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003; 31:459–465. PMID:
12665841.

74. Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003; 122:118–127. PMID:
12823353.

75. Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003; 102:1217–1223. PMID:
12714516.

76. Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005; 35:1187–1193. PMID:
15852025.

77. Rubegni P, Cuccia A, Sbano P, et al. Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol. 2005; 130:271–275. PMID:
16029456.

78. Bisaccia E, Palangio M, Gonzalez J, et al. Treatment of extensive chronic graft-versus-host disease with extracorporeal photochemotherapy. J Clin Apher. 2006; 21:181–187. PMID:
16607632.

79. Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006; 107:3074–3080. PMID:
16368882.

80. Berger M, Pessolano R, Albiani R, et al. Extracorporeal photopheresis for steroid resistant graft versus host disease in pediatric patients: a pilot single institution report. J Pediatr Hematol Oncol. 2007; 29:678–687. PMID:
17921848.
81. Perseghin P, Galimberti S, Balduzzi A, et al. Extracorporeal photochemotherapy for the treatment of chronic graft-versus-host disease: trend for a possible cell dose-related effect? Ther Apher Dial. 2007; 11:85–93. PMID:
17381528.

82. Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008; 112:2667–2674. PMID:
18621929.

83. Jagasia MH, Savani BN, Stricklin G, et al. Classic and overlap chronic graft-versus-host disease (cGVHD) is associated with superior outcome after extracorporeal photopheresis (ECP). Biol Blood Marrow Transplant. 2009; 15:1288–1295. PMID:
19747637.

84. Perotti C, Del Fante C, Tinelli C, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion. 2010; 50:1359–1369. PMID:
20113452.

85. Tzakis AG, Abu-Elmagd K, Fung JJ, et al. FK 506 rescue in chronic graft-versus-host-disease after bone marrow transplantation. Transplant Proc. 1991; 23:3225–3227. PMID:
1721416.
86. Kanamaru A, Takemoto Y, Kakishita E, et al. FK506 treatment of graft-versus-host disease developing or exacerbating during prophylaxis and therapy with cyclosporin and/or other immunosuppressants. Japanese FK506 BMT Study Group. Bone Marrow Transplant. 1995; 15:885–889. PMID:
7581086.
87. Carnevale-Schianca F, Martin P, Sullivan K, et al. Changing from cyclosporine to tacrolimus as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000; 6:613–620. PMID:
11128811.

88. Lee SJ, Wegner SA, McGarigle CJ, Bierer BE, Antin JH. Treatment of chronic graft-versus-host disease with clofazimine. Blood. 1997; 89:2298–2302. PMID:
9116272.

89. Couriel DR, Saliba R, Escalón MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005; 130:409–417. PMID:
16042691.

90. Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005; 11:47–55. PMID:
15625544.

91. Jurado M, Vallejo C, Pérez-Simón JA, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007; 13:701–706. PMID:
17531780.

92. Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant. 2011; 17:657–663. PMID:
20696263.

93. Chiang KY, Abhyankar S, Bridges K, Godder K, Henslee-Downey JP. Recombinant human tumor necrosis factor receptor fusion protein as complementary treatment for chronic graft-versus-host disease. Transplantation. 2002; 73:665–667. PMID:
11889452.
94. Giaccone L, Martin P, Carpenter P, et al. Safety and potential efficacy of low-dose methotrexate for treatment of chronic graft-versus-host disease. Bone Marrow Transplant. 2005; 36:337–341. PMID:
15968296.

95. Huang XJ, Jiang Q, Chen H, et al. Low-dose methotrexate for the treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005; 36:343–348. PMID:
15968295.

96. Inagaki J, Nagatoshi Y, Hatano M, Isomura N, Sakiyama M, Okamura J. Low-dose MTX for the treatment of acute and chronic graft-versus-host disease in children. Bone Marrow Transplant. 2008; 41:571–577. PMID:
18026150.

97. Robin M, Guardiola P, Girinsky T, et al. Low-dose thoracoabdominal irradiation for the treatment of refractory chronic graft-versus-host disease. Transplantation. 2005; 80:634–642. PMID:
16177638.

98. Akpek G, Lee SM, Anders V, Vogelsang GB. A high-dose pulse steroid regimen for controlling active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001; 7:495–502. PMID:
11669216.

99. Gilman AL, Chan KW, Mogul A, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000; 6:327–334. PMID:
10905770.

100. Shapira MY, Abdul-Hai A, Resnick IB, et al. Alefacept treatment for refractory chronic extensive GVHD. Bone Marrow Transplant. 2009; 43:339–343. PMID:
18850020.

101. Marcellus DC, Altomonte VL, Farmer ER, et al. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood. 1999; 93:66–70. PMID:
9864147.

102. Cho BS, Min CK, Eom KS, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009; 23:78–84. PMID:
18830253.

103. Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011; [Epub ahead of print].

104. Martin PJ, Storer BE, Carpenter PA, et al. Comparison of short-term response and long-term outcomes after initial systemic treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011; 17:124–132. PMID:
20601033.

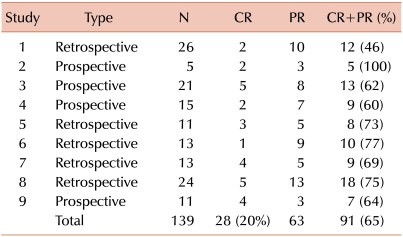
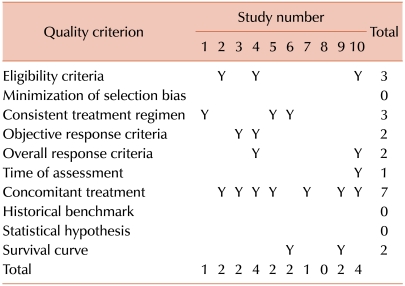
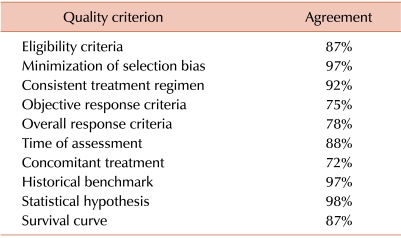
105. Cannistra SA. Phase II trials in journal of clinical oncology. J Clin Oncol. 2009; 27:3073–3076. PMID:
19451415.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download