This article has been
cited by other articles in ScienceCentral.
Abstract
Variant Burkitt-type translocation, t(8;22)(q24;q11), is very rare in plasma cell myeloma. We report a 51-year-old male patient with plasma cell myeloma, who showed t(8;22) (q24;q11). He suffered from pelvic pain for two months, and showed IgG, lambda type of monoclonal gammopathy (5.14 g/dL; 49.9% of protein). His bone marrow examination showed increased plasma cells (66.9% of all nucleated cells). Plasma cells (74.9% of all nucleated cells) and monoclonal spike (3.38 g/dL; 42.2%) persisted after three cycles of thalidomide and dexamethasone. Cytogenetic analysis showed complex chromosomal abnormalities: 44,XY,-1,t(2;5)(q33;q13),add(8)(q24.1),t(8;22)(q24.1;q11.2),add(10) (p15), der(11)t(1;11)(q21;p11.2),del(12)(p11.2p13),-13,-14,add(14)(q32),der(15)t(1;15)(p2 2;p11.2),-16,add(17)(q11.2),+21,+1-3mar[cp6]/46,XY[19]. To the best of our knowledge, this is the first report on plasma cell myeloma with a variant Burkitt-type t(8;22)(q24;q11) in the Korean patient. A review of 11 such cases in the literature, including the present case, implicated that plasma cell myeloma with t(8;22)(q24;q11) might be related to advanced stage and poor prognosis.
Go to :

Keywords: Plasma cell myeloma, t(8;22)(q24;q11), Variant, Burkitt
INTRODUCTION
Plasma cell myeloma is a B-cell clonal malignancy that is characterized by the accumulation of terminally differentiated plasma cells, which features monoclonal gammopathy, hypercalcemia, renal failure, anemia, and osteolytic bone lesions. Plasma cell myeloma accounts for 10% of hematological neoplasms, and it has a median survival of 2-3 years [
1,
2]. Abnormal karyotypes are observed in about 30-50% of plasma cell myelomas, and are usually associated with aggressive disease courses.
The t(8;14)(q24;q32) is classically related to Burkitt's lymphoma, and has been also seen in small noncleaved cell lymphoma, immunoblastic lymphoma, and, less frequently, in plasma cell myeloma [
3]. The
c-myc gene at 8q24 is juxtaposed to the immunoglobulin heavy chain (
IgH) gene at 14q32 and dysregulated. Variant Burkitt-type translocations, t(2;8)(p12;q24) or t(8;22)(q24;q11), are also specifically observed in Burkitt's lymphoma. These translocations, t(2;8) (p12;q24) and t(8;22)(q24;q11), involve light chain genes,
Igκ and
Igλ, respectively [
4].
We present a variant Burkitt-type translocation, t(8;22)(q24;q11), in a patient with resistant plasma cell myeloma. To the best of our knowledge, this is the first report on the variant Burkitt-type translocation, t(8;22)(q24;q11), related to plasma cell myeloma in Korea. We also reviewed 11 such cases, including the present case, to explore the clinical relevance of variant Burkitt-type translocation, t(8;22)(q24;q11), in plasma cell myeloma.
Go to :

CASE REPORT
In August 2010, a 51-year-old-male presented with right pelvic pain, occipital headache, weight loss, and weakness, which lasted for two months. He had visited a local hospital, and was referred to our hospital for further work-up under the suspicion of a hematologic malignancy. On physical examination, neither organomegaly nor lymphadenopathy was noted. His complete blood counts were: white blood cells, 6.3×109/L; hemoglobin, 8.9 g/dL; platelets, 233×109/L. The other laboratory findings were: total protein, 10.4 g/dL; albumin 2.5 g/dL; calcium, 8.6 mg/dL; blood urea nitrogen, 22.6 mg/dL; creatinine, 1.4 mg/dL; beta-2-microcroglobulin, 6.4 mg/L. Serum protein electrophoresis showed a monoclonal spike (5.14 g/dL; 49.9% of protein), which was confirmed as IgG, lambda type of monoclonal gammopathy by serum immunofixation. Radiographically, multiple osteolytic lesions were detected in right pelvic bone, spines, and skull.
Plasma cells were increased on the bone marrow aspirate smear (66.9% of all nucleated cells), and showed dispersed nuclear chromatin, high nuclear/cytoplasmic ratio, and often prominent nucleoli (
Fig. 1). The patient was diagnosed as having plasma cell myeloma. Flow cytometric immunophenotyping and cytogenetic analysis could not be performed at diagnosis, because the bone marrow study was performed at the previous hospital.
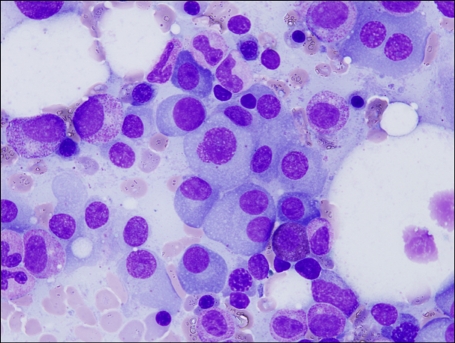 | Fig. 1Bone marrow aspiration smear showing increased plasma cells (Wright-Giemsa stain, ×1,000). 
|
After three cycles of thalidomide and dexamethasone, monoclonal spike (3.38 g/dL; 42.2%) was persistent, and plasma cells were counted up to 74.9% of all nucleated cells on the follow-up bone marrow aspirate smear. Flow cytometric immunophenotyping demonstrated an expanded population of abnormal plasma cells with CD19 negativity and positivity for the followings: CD38, 60%; CD138, 58%; CD56, 60%. Flow cytometric immunotyping for CD20, CD10 and light chain were not performed. Cytogenetic analysis showed a complex karyotype: 44,XY,-1,t(2;5)(q33;q13),add(8)(q24.1),t(8;22)(q24.1;q11.2),add(10)(p15),der(11)t(1;11)(q21;p11.2),del(12)(p11.2p13),-13,-14,add(14)(q32),der(15)t(1;15)(p22;p11.2),-16,add(17)(q11.2),+21,+1-3mar[cp6]/46,XY[19] (
Fig. 2) [
5]. Under the diagnosis of stable disease, he has received 1 cycle of bortezomib and dexamethasone, and has been clinically stable.
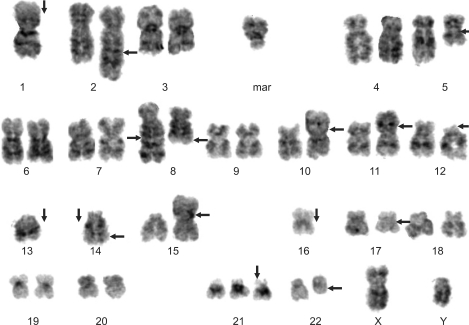 | Fig. 2Karyogram of follow-up bone marrow cells by Giemsa banding technique. 44,XY,-1,t(2;5) (q33;q13),add(8)(q24.1),t(8;22)(q24.1;q11.2),add(10)(p15),der(11)t(1;11)(q21;p11.2),del(12)(p11.2p13),-13,-14,add(14)(q32),der(15)t(1;15)(p22;p11.2),-16,add(17)(q11.2),+21,+1-3mar[cp6]. 
|
Go to :

DISCUSSION
Complex karyotypes are seen in 30 to 50% of plasma cell myeloma. Abnormal karyotypes are more often seen in advanced diseases, and are associated with increasing proliferative activity of malignant cells and poor outcome [
1,
6]. Poor-risk genetic abnormalities, such as the presence of t(14;16), t(14;20), del(13), t(4;14), and del(17p), are associated with significantly shorter event-free survival, overall survival, and duration of response [
6].
In this report, we have identified t(8;22)(q24;q11), a variant of t(8;14), in a patient with plasma cell myeloma. The break-points at 8q24 are localized within 300 kb downstream of the
c-myc gene, and
c-myc rearrangement, especially through t(8;14), is a major event in the oncogenesis of plasmacytomas [
7]. As a result of t(8;14) or its variants, the oncogene
c-myc is juxtaposed into the immunoglobulin gene locus [
8].
C-myc rearrangements were observed significantly more often in patients with β2-microglobulin levels above 3 mg/L [
9].
The variant Burkitt-type translocation, t(8;22)(q24;q11), has been reported in a limited number of cases, and only 18 such cases are found in the literature [
4,
8,
10-
15]. Among these 18 cases, eight cases were reported only with the results of fluorescence
in situ hybridization (FISH), without detailed cytogenetic and clinical information [
9]. The other 11 cases with variant Burkitt-type t(8;22), including our case, are summarized in
Table 1. Only two of them were reported to be alive, and all the cases with t(8;22)(q24;q11) had complex chromosomal abnormalities.
Table 1
Summary of 11 cases with plasma cell myeloma and t(8;22)(q24;q11).
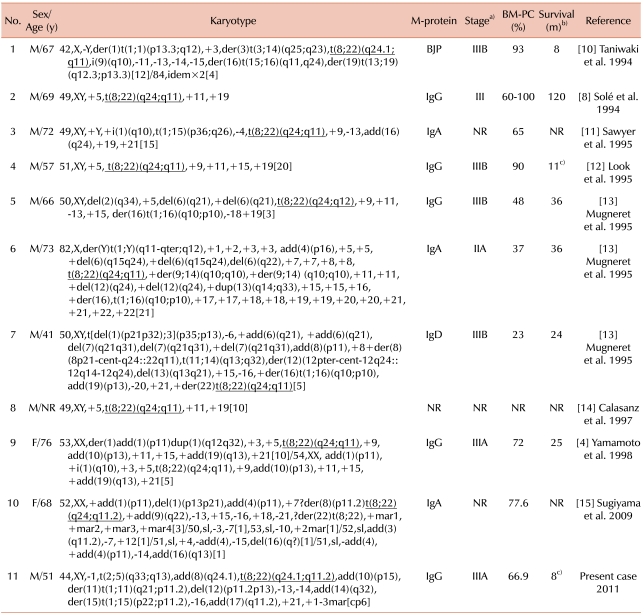

The presence of an abnormal karyotype is known to be related to a worse prognosis in plasma cell myeloma [
4]. Because all the reported cases with variant Burkitt-type t(8;22) concurrently showed complex karyotypic abnormalities, it is uncertain whether the variant Burkitt-type t(8;22) itself has prognostic or clinicopathologic implications or not. Further accumulation of cases with clinical details would be necessary to answer this question. In the present case, the initial chromosome study was not available, and the follow-up study was done within 84 days. Considering the other reported cases, we assume that the complex karyotype, including t(8;22), had been present since the initial diagnosis.
In summary, we report a variant Burkitt-type translocation, t(8;22)(q24;q11), in a patient with resistant plasma cell myeloma. A review of the literature implicated that t(8;22)(q24;q11) might be observed in plasma cell myeloma as a part of complex chromosomal abnormalities rather than a solitary cytogenetic abnormality. Consequently, plasma cell myeloma with t(8;22)(q24;q11) might be related to advanced stage and poor prognosis. Further accumulation of such cases is needed to delineate the clinical relevance of t(8;22)(q24;q11) in plasma cell myeloma.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download