Abstract
Background
Although a marked proportion of thalassemic patients acquire Torque teno virus (TTV) through blood transfusion, its clinical importance is unclear. This study was designed to investigate the clinical importance of TTV infection in thalassemic patients with and without hepatitis C virus (HCV) co-infection in Iran.
Methods
In this case-control study, 107 thalassemic patients on chronic transfusion and 107 healthy individuals were selected. According to HCV and TTV infection status (detected by semi-nested PCR), patients were categorized into 4 groups: TTV and HCV negative, TTV positive, HCV positive, and TTV and HCV positive. Blood ferritin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels in these 4 groups were assessed.
Results
Approximately half of the thalassemic patients (50.5%) and 27.1% of controls had TTV infection. Thalassemic patients had a greater chance of TTV infection compared to the control group with a sex-adjusted OR of 4.13 (95% CI=2.28-8.13). The increased levels of ALT, AST, and ferritin in the TTV and HCV-infected group were not significantly different from those in the TTV and HCV negative group. Co-infection with TTV and HCV did not significantly increase ALT, AST, and ferritin levels compared to infection with TTV alone.
Go to : 
Due to their dependency on blood transfusions, thalassemic patients are prone to transfusion-associated hepatitis, as a consequence of transfusion-related iron overload and exposure to viruses, which may cause hepatitis. The incidence of transfusion-related hepatitis in these patients has been substantially reduced since the implementation of blood screening for hepatitis B virus (HBV) and hepatitis C virus (HCV) nucleic acids and antibodies. However, a considerable proportion of thalassemic patients have raised levels of serum alanine aminotransferase (ALT), without a known causative hepatitis virus [1, 2].
Initially, hepatitis G virus (HGV) was presumed to be the causative agent of transfusion-associated non-A-C-hepatitis; however, this was not confirmed in subsequent studies. In 1997, Nishizawa and co-workers isolated a novel single-s tranded DNA virus, designated TT virus (TTV), from patients with non A-E transfusion-acquired hepatitis, and which appeared to be associated with non A-G post-transfusion hepatitis [3]. This virus was renamed Torque teno virus and was classified as a species of the Anellovirus genus in an unassigned family that is most closely related to the Circoviridae [4]. TTV infects the general population and particularly those at risk of parenteral exposure [5-8].
Transfusion-dependent patients, such as those with thalassemia, are more prone to carry TTV and most infections (80%) are of mixed genotypes [2, 9]. The prevalence of TTV in thalassemic patients varies in different studies and seems to be dependent on the diagnostic technique. Kondili et al. used two different sets of primers to detect TTV prevalence in 37 pediatric and young adult patients with thalassemia. The first primer set found TTV in 73% of thalassemic patients, while the second set found TTV DNA in 100% of the patients [10]. TTV infection can be detected by polymerase chain reaction (PCR), in situ hybridization, and specific antibodies to TTV [11]. Most TTV infections appear to occur parenterally but a substantial proportion of asymptomatic individuals never exposed to blood-borne agents and the high prevalence of TTV, even among healthy children, implicate the fecal-oral route of transmission as being potentially important [5, 12-14].
However, studies have not yet been able to confirm TTV as a primary cause of post-transfusion hepatitis, since most TTV-positive (TTV+) patients remain asymptomatic, and those progressing towards chronic liver disease are invariably co-infected with either HBV or HCV [5]. On the other hand, considering the fact that thalassemic patients are prone to acquiring various genotypes of TTV as a consequence of their life-long dependence on repeated blood transfusions, the importance of elucidating the potential role of this virus, if any, in development of clinical disease is highly important [2]. Hence, although a significant proportion of thalassemic patients acquire TTV by transfusion and some have co-infection with HCV, it remains uncertain if HCV and TTV co-infection leads to more severe hepatitis compared to TTV infection alone [15-20]. The clinical outcome of TTV in thalassemic patients is controversial, and there have been no previous studies in this regard in Iran. Thus, the current study is designed to investigate the prevalence and clinical importance of TTV infection in thalassemic patients with and without HCV co-infection in Iran.
Go to : 
In this case-control study, a total of 107 thalassemic patients, who received regular blood transfusions at the Thalassemia Center of Mofid Childrens' Hospital, Tehran, Iran, were selected as the patient group. One hundred and seven healthy volunteers were categorized as the control group. First, TTV DNA was assessed for all the patients and controls, and according to the HCV and TTV infection test results, cases were categorized into four groups: both TTV and HCV negative (TTV-HCV-), TTV positive alone (TTV+), HCV positive alone (HCV+), and TTV and HCV positive (TTV+HCV+). Blood ferritin, AST, and ALT levels in these 4 groups were assessed in order to determine the clinical outcome of TTV and HCV infections. In standard donor-screening tests, all the samples were negative for HIV, human T cell leukemia viruses, and HBV. Informed consent for serological studies was obtained from the patients or their parents if the patients were under 18 years of age. The study protocol was approved by the ethics committee of Tehran University of Medical Sciences (TUMS).
Serum ALT and AST levels were measured using an automated analyzer and values higher than 50 and 40 IU/L, respectively, were considered to be abnormal. Serum ferritin was determined by ELISA (IMx ferritin assay; Abbott Division Diagnostici, Rome, Italy) and because of its high plasma levels in thalassemic patients, values higher than 3,000 ng/mL were considered to be hyperferritinemia. Anti-HCV status was determined by a commercially available second generation enzyme-linked ELISA (hepatitis C II; Abbott Laboratories, North Chicago, IL, USA) and hepatitis B surface antigen (HBsAg) was determined by radioimmune assay (Abbot Laboratories, North Chicago, IL, USA). HCV RNA was detected by RT-PCR as described previously [21-23].
Semi-nested PCR was used to detect serum TTV DNA. Specifically, serum DNA purified from an equivalent of 7 µL of serum was amplified using the following PCR protocol in 9600 thermal cycler (Perkin-Elmer, Emeryville, CA, USA); 1 cycle at 95℃ for 9 min, 35 cycles at 94℃ for 30 s, 58℃ for 30 s, 72℃ for 45 s, 1 cycle at 72℃ for 7 min. The reaction mix contained 30 pmol of each primer (sense NG059: 5'-ACA GAC AGA GGA GAA GGC AAC ATG-3', antisense NG063: 5'-CTG GCA TTT TAC CAT TTC CAA AGT-3') and 2.5 U of AmpliTaq Gold (Perkin-Elmer, Emeryville, CA) in a 50 µL reaction volume. Under the same conditions, the second round of PCR was performed using a semi-nested primer set (sense NG061; 5'-GGC AAC ATG TTA TGGATA GAC TGG-3', antisense NG063) and 5 µL of the amplification product. Multiple positive and negative controls were included in each PCR assay. PCR products were analyzed on 2% agarose gel with ethidium bromide staining. For all positive samples of TTV DNA, separate assays were performed and sequences of PCR products were confirmed by automated sequencing on ABI 373 sequencer (Perkin-Elmer, Foster City, CA, USA).
Results were reported as the mean±standard deviation (SD) for quantitative variables and percentages for categorical variables. We categorized AST, ALT and ferritin levels into normal and abnormal groups using the clinical ranges described above. The groups were compared using Student's t-tests for continuous variables and chi-square tests (or Fisher's exact test, if required) for categorical variables. Statistical significance was based on two-sided design-based tests with P≤0.05 being considered significant. All statistical analyses were performed using SPSS version 13 (SPSS Inc., Chicago, IL, USA) for Windows.
Go to : 
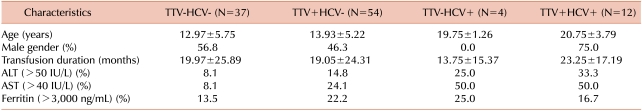
In this study, 107 thalassemic patients (51.4% male) with a mean age of 14.61±5.96 years and an average transfusion duration of 19.64±23.60 months were selected as the patient group. A representative sample of 107 healthy volunteers (33.6% male) with a mean age of 13.13±6.37 years were selected as the control group. According to their HCV and TTV infection results, the patient group was divided into four subgroups (TTV-HCV-, TTV+, HCV+, and TTV+HCV+). TTV infection was found in 27.1% of controls. Nearly two-thirds (65.4%) of thalassemic patients carried a virus of which approximately half (50.5%) had TTV infection alone and 11.2% had both TTV and HCV infection. HCV infection alone was found in 4 patients (3.7%).
Demographic and clinical data of the 4 groups is shown in Table 1. Seventy-five percent of TTV+HCV+ patients and 46.3% of TTV+ patients were male. Patients who were co-infected with TTV and HCV were older when compared to those infected with TTV alone (20.8±3.8 vs. 14.0±5.8 years, P<0.001) and tended to have been undergoing blood transfusions for longer (23.3±17.2 vs. 19.1±24.3 months, P=0.58), although this difference was not statistically significant. Abnormal values of serum ALT (>50 IU/mL) and AST (>40 IU/mL) levels were more often found in TTV+HCV+ patients compared to the TTV+HCV- group (33.3% vs. 14.8% and 50.0% vs. 24.1%, respectively). This is in contrast to the incidence of hyperferritinemia (>3,000 ng/mL), which was observed more often in the TTV+HCV- group than in TTV+HCV+ patients, although was not statistically significant. An age >15 years, gender, or transfusion duration of >12 months were not correlated with the incidence of infection with TTV alone. However, there was a significant relationship between older age (>15 years) and TTV-HCV co-infection (OR=1.86, 95% CI=1.30-2.65), although a similar relationship was not found between gender or transfusion duration (>12 months) and TTV-HCV co-infection.
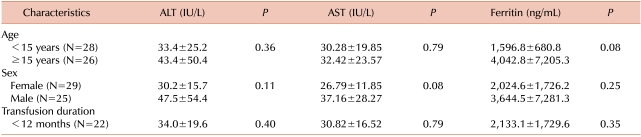
In order to evaluate the influence of age, sex, and transfusion duration on clinical outcome in TTV+ patients, we compared mean plasma levels of ALT, AST, and ferritin in these patients under these categories (Table 2). However, we found no significant differences in these plasma biochemical markers with increased age, transfusion duration, or gender (P>0.05).
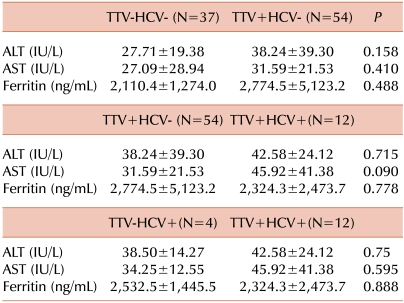
Thalassemic patients had a greater chance of TTV infection in comparison to the control group, with a pure OR of 3.93 (95% CI=2.16-7.13). This relationship was also confirmed even when we considered sex as a probable confounding factor (OR=4.13, 95% CI=2.28-8.13), with males being significantly more prevalent among TTV patients than uninfected controls (51.4% vs. 33.6%, respectively, P=0.012). In Table 3, the mean plasma levels of ALT, AST, and ferritin in the TTV-HCV-, TTV+HCV-, TTV-HCV+ and TTV+HCV+ groups are compared. The increased levels of ALT, AST, and ferritin observed in the TTV+HCV- group did not differ statistically from those of the TTV-HCV- group. Furthermore, co-infection with TTV and HCV did not significantly increase ALT, AST, or ferritin levels when compared with infection with either TTV or HCV alone.
Go to : 
The potential role and clinical importance of TTV infection in liver diseases, as a transfusion-transmitted agent in thalassemic patients and other transfusion-dependent diseases, has been discussed since the discovery of the virus in 1997. The prevalence of TTV varies among thalassemic patients from different nations. Approximately half of the patients (50.5%) and more than a quarter of controls (27.1%) were found to have TTV infection. Hsu et al. also found 27.0% of 122 healthy children in Taiwan to be infected with TTV [12]. In our study, thalassemic patients had a greater chance of TTV infection in comparison with the control group with a common OR of 4.31 (95% CI=2.28-8.13). This could reflect the greater importance of the parenteral route for virus transmission that has been noted in previous studies [5, 12, 24]. However, among infected subjects in the control group were healthy young children without any transfusion history or known diseases, suggesting a non-parenteral route of transmission for TTV. Epidemiological studies suggest that the main routes of TTV infection are parenteral, oral-fecal, and possibly salivary [14].
The prevalence of TTV among thalassemic patients varies in different studies and has been reported to be as high as 100% [10, 25-27], depending on diagnostic technique and specimen type. Chen et al. used peripheral blood mononuclear cells, plasma, saliva, and urine samples from 50 thalassemic patients for TTV detection. They found that all 50 patients had TTV in one or more specimens and that 16.0% of patients were positive for all specimen types [28].
In the current study, abnormal levels of ALT and AST were observed in a considerable proportion of TTV+ patients (14.8% and 24.1%, respectively) and in TTV+HCV+ patients (33.3% and 50.0%, respectively). Moreover, abnormal levels of these markers were also found in 8.1% of patients without TTV or HCV infection. The reason for this is not clear but might be explained by liver disease associated with transfusion-related iron overload, the presence of undiagnosed TTV genotypes, or other blood-borne agents. Studies by Hu and co-workers confirm our findings that there is a higher incidence of abnormal ALT and AST levels in TTV+ patients (33.6% and 36.7%, respectively) and TTV+HCV+ patients (56.6% and 70.0%, respectively) [24]. They concluded that ALT, AST, and ferritin levels were invariably lower in TTV- patients than in TTV+ patients and that there are significantly elevated ferritin and ALT levels with TTV-HCV co-infection compared to TTV infection alone [24]. In the current study, TTV+HCV- patients tended to have increased levels of ALT and AST compared to the TTV-HCV- group, as did TTV+HCV+ patients compared to the TTV+HCV- group, although these differences were not statistically significant. Some recent studies also demonstrated that co-infection with TTV and HCV in thalassemic patients does not alter the plasma profile of biochemical markers when compared with TTV infection alone [26]. The same pattern was also found for TTV+HCV+ patients compared with those infected with HCV alone. However, due to the low prevalence of HCV+ patients (4 patients), these findings should be considered with caution.
Assessing the severity of liver disease solely by measurements of plasma transaminase levels is inadequate. Histological data are also necessary, possibly with an estimated duration of virus infection. However, due to the lack of histological studies of patient tissues in the current study, we were not able to address this issue. In our study, to enhance cost-effectiveness ALT, AST, and ferritin levels were only measured in the control group. Regardless of whether TTV is a cause of liver disease in thalassemic patients, pathogenic mechanisms of the virus need to be rapidly elucidated in order to develop new strategies to prevent transmission and for therapeutic intervention. Targeted longitudinal studies of TTV in the future will be helpful in this regard [29]. On the basis of our study, it can be concluded that TTV, despite being widely distributed among thalassemic patients, appears to have a negligible role in increasing the severity of liver disease, even when co-infection with HCV occurs.
Go to : 
References
1. Okamoto H, Mayumi M. TT virus: virological and genomic characteristics and disease associations. J Gastroenterol. 2001; 36:519–529. PMID: 11519830.

2. Chen BP, Rumi MG, Colombo M, et al. TT virus is present in a high frequency of Italian hemophilic patients transfused with plasma-derived clotting factor concentrates. Blood. 1999; 94:4333–4336. PMID: 10590078.

3. Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997; 241:92–97. PMID: 9405239.

4. Hino S. TTV, a new human virus with single stranded circular DNA genome. Rev Med Virol. 2002; 12:151–158. PMID: 11987140.

5. Poovorawan Y, Tangkijvanich P, Theamboonlers A, Hirsch P. Transfusion transmissible virus TTV and its putative role in the etiology of liver disease. Hepatogastroenterology. 2001; 48:256–260. PMID: 11268979.
7. Simmonds P. Transfusion virology: progress and challenges. Blood Rev. 1998; 12:171–177. PMID: 9745887.

8. Blejer JL, Salamone HJ. Is TT virus (TTV) a true hepatitis virus cause? Medicina (B Aires). 2000; 60:631–638. PMID: 11188907.
9. Okamoto H, Takahashi M, Nishizawa T, et al. Marked genomic heterogeneity and frequent mixed infection of TT virus demonstrated by PCR with primers from coding and noncoding regions. Virology. 1999; 259:428–436. PMID: 10388666.

10. Kondili LA, Pisani G, Beneduce F, et al. Prevalence of TT virus in healthy children and thalassemic pediatric and young adult patients. J Pediatr Gastroenterol Nutr. 2001; 33:629–632. PMID: 11740244.

11. Liweń I, Januszkiewicz-Lewandowska D, Nowak J. TT virus-characteristics, occurrence and routes of transmission. Przegl Epidemiol. 2002; 56:91–99.
12. Hsu HY, Ni YH, Chen HL, Kao JH, Chang MH. TT virus infection in healthy children, children after blood transfusion, and children with non-A to E hepatitis or other liver diseases in Taiwan. J Med Virol. 2003; 69:66–71. PMID: 12436479.

13. Davidson F, MacDonald D, Mokili JL, Prescott LE, Graham S, Simmonds P. Early acquisition of TT virus (TTV) in an area endemic for TTV infection. J Infect Dis. 1999; 179:1070–1076. PMID: 10191206.

14. Yzèbe D, Xueref S, Baratin D, Boulétreau A, Fabry J, Vanhems P. TT virus. A review of the literature. Panminerva Med. 2002; 44:167–177. PMID: 12094130.
15. Tokita H, Murai S, Kamitsukasa H, et al. High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J Med Virol. 2002; 67:501–509. PMID: 12115995.

16. Meng XW, Komatsu M, Goto T, et al. Clinical significance of TT virus in chronic hepatitis C. J Gastroenterol Hepatol. 2001; 16:202–208. PMID: 11207902.

17. Cleavinger PJ, Persing DH, Li H, et al. Prevalence of TT virus infection in blood donors with elevated ALT in the absence of known hepatitis markers. Am J Gastroenterol. 2000; 95:772–776. PMID: 10710073.

18. Yuki N, Kato M, Masuzawa M, et al. Clinical implications of coinfection with a novel DNA virus (TTV) in hepatitis C virus carriers on maintenance hemodialysis. J Med Virol. 1999; 59:431–436. PMID: 10534723.

19. Charlton M, Adjei P, Poterucha J, et al. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998; 28:839–842. PMID: 9731581.

20. Zein NN, Arslan M, Li H, et al. Clinical significance of TT virus infection in patients with chronic hepatitis C. Am J Gastroenterol. 1999; 94:3020–3027. PMID: 10520863.

21. Ni YH, Chang MH, Lue HC, et al. Posttransfusion hepatitis C virus infection in children. J Pediatr. 1994; 124:709–713. PMID: 8176556.

22. Lai MW, Chang MH, Hsu HY. Non-A, non-B, non-C hepatitis: its significance in pediatric patients and the role of GB virus-C. J Pediatr. 1997; 131:536–540. PMID: 9386654.

23. Chen HL, Chang MH, Ni YH, Hsu HY, Kao JH, Chen PJ. Hepatitis G virus infection in normal and prospectively followed posttransfusion children. Pediatr Res. 1997; 42:784–787. PMID: 9396558.

24. Hu YW, Al-Moslih MI, Al Ali MT, et al. Clinical outcome of frequent exposure to Torque Teno virus (TTV) through blood transfusion in thalassemia patients with or without hepatitis C virus (HCV) infection. J Med Virol. 2008; 80:365–371. PMID: 18098140.

25. Sampietro M, Tavazzi D, Martinez di Montemuros F, et al. TT virus infection in adult beta-thalassemia major patients. Haematologica. 2001; 86:39–43. PMID: 11146569.
26. Ozyürek E, Ergünay K, Kuskonmaz B, et al. Transfusion-transmitted virus prevalence in Turkish patients with thalassemia. Pediatr Hematol Oncol. 2006; 23:347–353. PMID: 16621777.
27. Erensoy S, Sayiner AA, Türkoğlu S, et al. TT virus infection and genotype distribution in blood donors and a group of patients from Turkey. Infection. 2002; 30:299–302. PMID: 12382090.

28. Chan PK, Chik KW, Li CK, et al. Prevalence and genotype distribution of TT virus in various specimen types from thalassaemic patients. J Viral Hepat. 2001; 8:304–309. PMID: 11454183.

29. Dhenain M, Boulétreau A, Bourguignon F, et al. The TT virus: review of the literature. Clin Invest Med. 2000; 23:355–365. PMID: 11271001.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download