INTRODUCTION
Allogeneic hematopoietic stem-cell transplantation (HSCT) is a well-established curative therapy for the treatment of lymphohematopoietic and congenital metabolic disease. Because there is a lack of HLA-identical related donors in approximately 75% of the cases, during the past few years, an increasing number of HSCTs have been performed using HLA-matched unrelated donors (URDs) [
1]. Employment of URD introduces a number of questions and problems in donor selection that do not occur in transplantation from an HLA-identical sibling donor. The precise impact of HLA mismatching on HSCT outcomes remains unclear, because studies on the relative importance of various loci involved have yielded different results. Nearly every HLA locus has been reported to influence the outcome of URD HSCT; however, conflicting data have been obtained [
2-
4]. In American patients, a single mismatch (Mm) at HLA-B or -C was better tolerated than those at HLA-A or -DRB1 [
5]. In Japanese patients, however, the presence of a HLA-A or -B Mm significantly reduced survival, whereas a Mm at HLA-C or -DRB1/DQB1 did not [
6]. Molecular typing techniques for all HLA loci have demonstrated that serological matching is insufficient to ensure an allelic match [
7,
8]. High-resolution DNA-based typing of HLA alleles has markedly improved donor selection accuracy, resulting in improved HSCT patient outcomes. However, the relative importance of antigenic and allelic Mms remains unclear [
5,
9].
The impact of HLA Mms on disease outcomes in Korean children has not been determined, and the relationship between Mms and disease status has not been explored. We, therefore, retrospectively assessed the impact of high-resolution donor-recipient matching results at the HLA-A, -B, -C, and -DR loci on patient outcomes in 142 Korean children treated with URD HSCT.
Go to :

MATERIALS AND METHODS
1. Patients
This study included 142 patients aged ≤18 years, who received HSCT from URDs at 4 medical centers (Asan Medical Center, Seoul National University Hospital, Samsung Medical Center, and National Cancer Center) in Korea between April 2003 and September 2009. The median follow-up duration was 22 months (range, 1-78 months).
All patient-donor pairs were fully typed for HLA-A, -B, -C, and -DR alleles. Details of the study population are shown in
Table 1. Underlying malignant diseases included acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), non-Hodgkin's lymphoma (NHL), myelodysplastic syndrome (MDS), and chronic myeloid leukemia (CML). Nonmalignant diseases included bone marrow failure, immunodeficiency, hemophagocytic lymphohistiocytosis, and metabolic disease. Patients with hematological malignancies were divided into low-risk and high-risk subgroups based on disease status at transplantation. Low-risk individuals had ALL or AML in first complete remission (CR), MDS with either refractory anemia or refractory anemia with ringed sideroblasts subtypes, or CML in first chronic phase (CP). All other hematologic malignancies were considered high risk. Seventy-one patients were classified as low-risk and 38 as high-risk.
Table 1
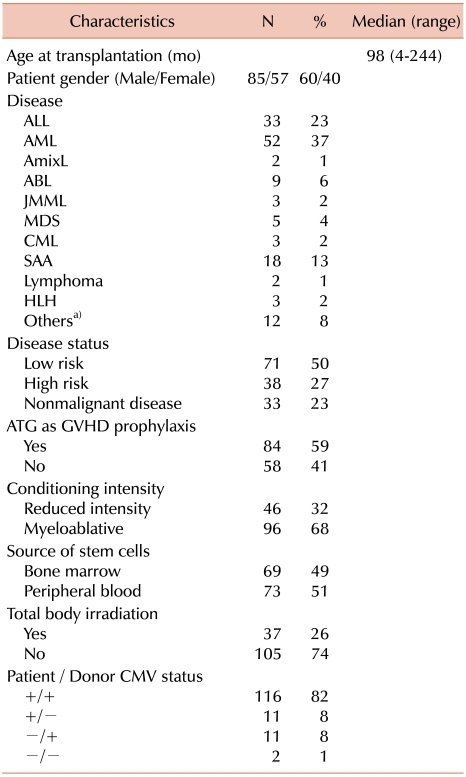

Stem cell sources included bone marrow in 69 (49%) and granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood in 73 (51%) patients. Recipients of cord blood were excluded in this study, because HLA Mms may have a different impact in this patient population.
2. HLA typing and matching
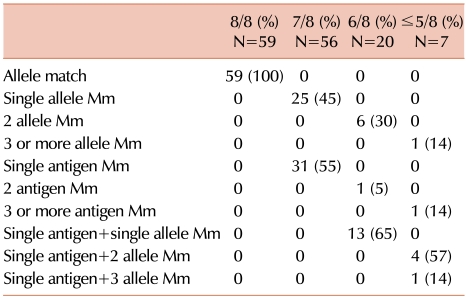
All donors and recipients were HLA-typed both serologically and using PCR by using sequence-specific primers (SSPs), and all donor-recipient pairs were fully typed for HLA-A, -B, -C, and -DR using high-resolution molecular typing. All Mms were classified according to the loci involved and whether they were detected at the low- or high-resolution level.
3. Definitions of outcomes
The primary outcome was overall survival (OS), defined as the time from graft infusion to death from any cause. Treatment-related mortality (TRM) was defined as death during continuous CR of the primary disease. Secondary endpoints included neutrophil engraftment, defined as timing of the first 3 consecutive days on which a patient had neutrophil counts >0.5×10
9/L, the cumulative incidence of grades II-IV acute graft-versus-host disease (aGVHD), and the cumulative incidence of relapse in patients with malignant disease. aGVHD was diagnosed and graded using established criteria [
10]. Patients were considered evaluable for engraftment, if they survived for at least 21 days after HSCT. Relapse-related death was defined as death caused by relapse after HSCT, regardless of any further treatment for relapse.
4. Statistical analysis
Continuous variables are expressed as median (range), whereas categorical variables are expressed as proportions and/or percentages. Descriptive statistical analysis was performed to compare patient baseline and post-transplantation characteristics. Two-sided Fisher's exact test was used in 2×2 table analysis. OS curves were calculated using Kaplan-Meier method and compared using the log-rank test. Cumulative incidence curves for TRM and relapse with or without death were constructed to reflect the time to relapse and time to transplant-related death as competing risks. Cumulative incidences were compared using Gray's test. Both analyses were performed using Cox regression for OS, TRM and relapse-related death, and logistic regression for aGVHD and engraftment. Variables considered included HLA match, patient age, donor-recipient gender match, disease status, stem cell source, and intensity of conditioning. All reported P-values are 2-sided, and P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 18.0 software program or R 2.10.1.
Go to :

DISCUSSION
Extensive recent research has shown that mismatches at each HLA locus can affect clinical outcomes of patients undergoing URD HSCT [
11-
13]. The role of HLA on HSCT outcomes had not previously been assessed in Korean children; thus, we evaluated the effects of patient-donor HLA compatibility in these children. This is the first multicenter study to analyze the relative clinical importance of HLA matching on major transplantation outcomes in Korean children. Several important results emerged from our analysis.
First, there were no differences in survival, aGVHD, and relapse when single allele and single antigen Mms were compared, in contrast to the common perception that allele Mms are better tolerated than are antigen Mms. Previous studies have reported conflicting results, with single allele and antigen Mms showing either equivalent [
4,
5,
9] or different [
14] effects on outcomes. Although particular high-resolution Mms have been regarded as more permissive than their low-resolution counterparts, data on pooled high-resolution Mms showed that adverse effects on transplantation outcomes were detectable [
14]. Our results suggest that both low- and high-resolution Mms should be considered equivalent when determining optimal donor-patient HLA matches. However, our inability to detect significant differences may be because the donor-recipient population in this study was relatively small.
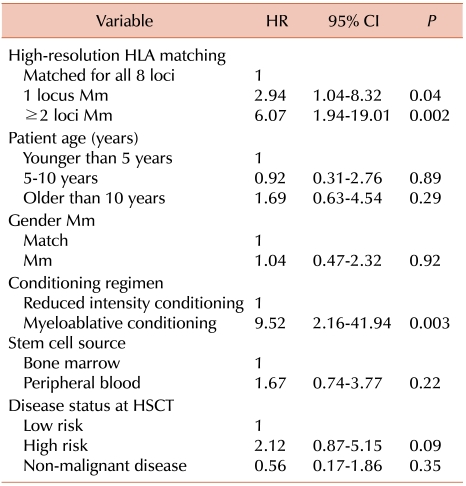
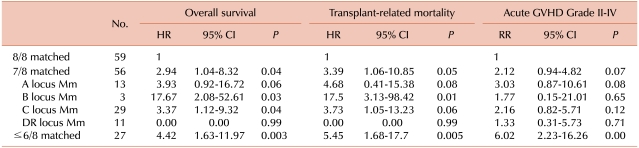
Our results demonstrate the negative effects of a single Mm on survival. Some Mms exhibit worse transplant outcomes than other Mms. A recent National Marrow Donor Program (NMDP) study [
14] found that single Mms at HLA-A, -B, -C, and -DR loci had similar adverse effects on mortality, and other reports have described a deleterious effect of HLA-C Mms on survival [
3,
14,
15]. In this study, we found that single Mms at HLA-B and -C, but not at HLA-A and -DR, were associated with significantly lower survival rates, compared to those in patients with 8 matched alleles. These findings agree with the hypothesis that Mms at some loci are better tolerated than those at the others. As the strong linkage disequilibrium between HLA-B and -C results in frequent coordinate matching, information on both loci is of importance in determining optimal donor-patient HLA matches [
16]. Moreover, HLA-A Mms are associated with a significantly higher risk of grades III-IV aGVHD. Although reports have yielded different results when the association between HLA disparity and aGVHD were compared, our findings confirm that HLA-A Mms have significant adverse effects on severe aGVHD [
14].
An elevated number of HLA Mms also increased the risk of post-transplantation complications, including poorer overall survival, TRM, and aGVHD incidence and severity. In this study, each additional Mm was associated with a 17-18% absolute decrease in survival. Most previous studies have shown that multiple Mms increase mortality and the risk of post-transplantation complications.
Notably, the impact of HLA Mms differed among patients at various stages of disease. Some previous studies have reported that high-risk disease before HSCT had a greater absolute impact on survival than did HLA mismatching [
5,
9,
15]. In this study, however, the impact of single HLA Mms on survival was more detrimental in patients with high- compared to low-risk malignancies. These differences may be due to higher incidence of grade III-IV aGVHD (
P=0.05) and the use of a myeloablative conditioning regimen (
P=0.01) in high-risk malignancies compared to those in low-risk malignancies; differences may also reflect higher intensities of immunosuppression and the tendency to have an infection. We also found that TRM rates were proportional to the extent of HLA mismatching in both the high- and low-risk disease categories; thus, the greater impact of single Mms on survival in patients with high- rather than low-risk disease is likely attributable to augmentation in patients with higher-risk disease of the negative effect of HLA disparity associated with TRM. Because the major cause of TRM in this study was sepsis, early identification and proper treatment of infections is imperative, if HSCT is performed in a patient with high-risk disease using an incompletely matched donor.
The acceptable Mm level remains controversial. This is important in donor selection, when a fully matched donor is not available, and a choice must be made between accepting a mismatched donor, searching for other sources of stem cells or a haploidentical donor, and completely avoiding HSCT. Guidelines of the International Histocompatibility Working Group (IHWG) state that a full application of the rules governing the acceptability of HLA Mms should include side-by-side evaluation of ethnically and racially diverse transplantation populations (
www.ihwg.org). Analysis of very large numbers of pairs, characterized at high resolution and for whom complete clinical data are available, is of crucial importance in addressing locus- and allele-specific rules for mismatching [
17]. The apparent discrepancy between our results and those of other studies may be attributable to both study power and ethnicity. A discrepancy between Korean and other patients may reflect disparities in the HLA and non-HLA genetic backgrounds of the more homogeneous Korean population compared with more heterogeneous populations. Some HLA alleles and haplotypes are distributed at different frequencies among various racial/ethnic groups [
11]. Thus, specific Mms among HLA-loci may be better tolerated within certain ethnic groups. For example, a study in Japan suggested that discrepancies of responsible HLA locus for aGVHD between ethnically diverse HSCT may be explained by the proportions of nonpermissive Mm combinations in each HLA locus [
18].
Although several studies have found lower relapse risks, of the graft-vs.-leukemia effect in patients with Mms at multiple loci or at a specific locus [
3,
19], we did not find an association between HLA disparity and relapse; there may be an association of donor/patient HLA mismatching with low rate of discontinuation of immunosuppression and prolonged immunosuppressive therapy for GVHD compared to HLA-matched transplantation. However, as other studies did not observe an effect of HLA disparity on relapse, further investigations should be conducted to address this issue [
4,
5].
Our present study had several limitations. First, our patient sample was relatively small and heterogeneous, and transplantation protocols were not uniform. Only 3 patients had HLA-B Mm; therefore, results should be interpreted with caution. However, some analyses regarding HLA-B Mm show a
P<0.05. Second, subset comparisons have specific strengths, but confounding by Mms at other loci is avoided. Thus, this method may overlook a functional role of HLA-A, -B, -C, or -DR mismatching that operates only in combination with one or more Mms. Membership in groups with double and triple Mms was too low to permit statistical analysis. Third, we did not analyze the effect of HLA-DP and -DQ, which generally showed additive effects when additional HLA Mms were present at other loci [
6,
20].
In summary, we showed that outcomes in single-allele Mm patients are not equivalent to those in patients with 8/8 matches. Disparity in HLA class I status, regardless of antigen or allele Mm, had adverse effects on survival and grade III-IV aGVHD, whereas HLA class II disparities had little impact on transplantation outcomes. An increased number of HLA Mms was associated with higher rates of post-transplantation complications, including poorer survival and increased aGVHD incidence or severity. HLA disparities impacted survival differently when recipients had low- or high-risk malignancies. Physicians overseeing HSCT for high-risk patients using HLA-mismatched donors should avoid and treat infections and associated complications to reduce TRM.
This study provides useful information regarding the significance of HLA matching on outcomes of Korean pediatric patients undergoing URD HSCT, thus assisting physicians in their search for a suitable donor. Further investigations, including analyses of larger cohorts, are warranted to confirm the effects of HLA Mms on outcomes of patients undergoing HSCT.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download