Abstract
Imatinib mesylate (IM) is used to treat a wide range of diseases, including Philadelphia chromosome-positive chronic myeloid leukemia (CML), on account of its high tolerability and low incidence of minor adverse events. Hemorrhage is thought to be a rare complication of IM. Recently, IM has been associated with reduced α2-plasmin inhibitor and platelet dysfunction. We report here the case of a 33-year-old female patient with CML who experienced subdural hematoma after an incremental increase in IM dosage due to a loss of complete molecular response. This case indicates that physicians should be alert to this atypical cause of headache in patients taking high-dose IM.
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by the presence of the Philadelphia chromosome, which results from the reciprocal chromosomal translocation t(9;22)(q34;q11) [1]. Imatinib mesylate (IM, Gleevec; Novartis, Switzerland) is a potent and selective tyrosine kinase inhibitor that acts on Bcr/Abl, the protein product of the Philadelphia chromosome. This drug has revolutionized the treatment of CML and is now a standard initial treatment for CML [2]. In clinical trials using IM, common side effects of nausea, emesis, diarrhea, periorbital edema, fluid retention, and myelosuppression have been documented. Nevertheless, phase I and II studies have found IM to be well tolerated with most of the side effects being minor [3, 4].
We report here an unusual case of subdural hematoma in a patient with CML treated with high-dose IM. To the best of our knowledge, this adverse event has not been previously reported in Korea.
A 33-year-old woman was diagnosed in September 2004 with chronic phase Philadelphia chromosome-positive CML, and IM was initiated at a dose of 400 mg daily. A complete cytogenetic response (CCyR) was achieved after 3 months of therapy. A complete molecular response (CMR) was obtained after 12 months of therapy (in September 2005).
In April 2009, a peripheral blood sample showed a positive result for BCR-ABL fusion transcript, as well as a loss of CMR. Cytogenetic examination of a bone marrow sample showed sustained CCyR. The treatment dosage was increased to 600 mg per day because of the loss of CMR and increased again to 800 mg per day 1 month later owing to the detection of an M351T IM-resistance mutation. In August 2009, follow-up laboratory findings showed a slightly decreased quantity of the BCR-ABL fusion transcript. However, the dose was reduced to 600 mg per day in September 2009 because the patient began to experience intolerable headaches. Fourteen days after her last outpatient appointment, the patient complained of severe headache with nausea and vomiting. She also developed a visual field defect, a black spot in her right eye and was accordingly referred to local ophthalmology clinics. On ophthalmoscopic examination, optic disc swelling and hemorrhage were detected. She had no history of prior traumatic events or of anti-coagulation medication.
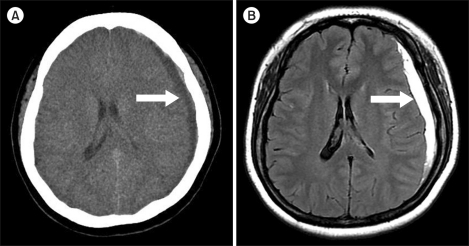
She was subsequently transferred to our emergency center. On general examination, her blood pressure was found to be 150/90 mmHg and her heart rate was 80/min. Lung sounds were clear and heart sounds were normal. A neurologic examination was also normal. Laboratory data showed the following: hemoglobin, 11.4 g/dL; hematocrit, 33.6%; white blood cell count, 8.3×109/L (segmented neutrophils, 63.2%; lymphocytes, 25.2%; monocytes, 5.9%; eosinophils, 5.5%; basophils, 0.2%); platelets, 300×109/L; prothrombin time, 11.1 sec (range, 9.8-13.5 sec); aPTT 26.3 sec (range, 21-32.4 sec); fibrinogen, 258 (range, 200-400 mg/dL); FDP, 2 µg/mL (range, <5 µg/mL); D-dimer, 0.2 µg/mL (range, <1 µg/mL); serum sodium, 137 mEq/L; potassium, 3.7 mEq/L; chloride, 103 mEq/L; blood urea nitrogen, 8 mg/dL; serum creatinine, 0.9 mg/dL; total protein, 6.9 mg/dL; and albumin 4.3, mg/dL. Urinalysis was normal. However, a subacute subdural hematoma was detected in the left frontotemporoparietal convexity on brain computed tomography and magnetic resonance images (Fig. 1). She was admitted to the department of neurosurgery. IM was discontinued for 2 days and then maintained at a dose of 400 mg daily, and the patient was managed conservatively for 1 week. As a consequence of this treatment, her headache and visual symptoms were relieved. The IM dose was subsequently increased to 500 mg daily 1 month after the hemorrhagic event and increased again to 600 mg daily after a further month. In November 2009, she achieved CMR, as confirmed by RT-PCR. She is currently on a maintenance dose of IM 600 mg daily and has experienced no significant adverse events.
The incidence of subdural hematoma in the general population is estimated to be 1-15 cases per 100,000 patients [5]. The data indicate that for patients treated with IM, central nervous system hemorrhage occurs in 5% of those in blast crisis, 1% in the accelerated phase, and 0.6% in the chronic phase [6].
Our patient developed subdural hematoma at an IM dose of 800 mg daily without thrombocytopenia or coagulation abnormalities. IM pharmacokinetic analysis in the IRIS study showed that hemorrhagic complications were not increased by higher IM trough levels at 400 mg daily during steady state [7]. That study showed that the frequency of grade 3 or 4 hemorrhagic events was 0-1.1% during the first 3 months and 1.1-2.2% during the 5-year treatment period according to trough level category. Song et al. [8] reported that 7 of 121 advanced CML patients treated with IM initially at 600 mg per day were diagnosed with non-traumatic subdural hematomas. All patients had subdural hematomas that developed at a median of 10 weeks (1-48 weeks) after the start of therapy. In our patient, subdural hematoma developed at 20 weeks after increasing the IM dose to 800 mg daily. However, there is no definite evidence that a high IM trough level was associated with the development of major hemorrhagic events.
Interestingly, Radaelli et al. [9] reported that 10 (11%) of the 87 patients who had taken IM at a dose of 300-600 mg daily for a minimum of 3 months developed unilateral or bilateral conjunctival hemorrhage. No other hemorrhagic events were observed during follow-up; the exception being conjunctival hemorrhage recurrence in 6 cases (7%). Therefore, the authors decided not to stop using IM if only conjunctival hemorrhage occurred in the absence of other major hemorrhagic events. Our patient discontinued the drug for 2 days and was then put on a maintenance dose of 400 mg daily for 1 month. This was because the patient had previously experienced no adverse events at a dose of 400 mg daily and had developed the subdural hematoma at a dose of 800 mg. We monitored the patient closely for additional hemorrhagic events and increased the dose of IM to 600 mg daily to prevent CML progression. Follow-up brain MRI showed complete resolution of the subdural hematoma after 1 month at a dose of 600 mg daily.
Matsue et al. [10] reported decreased levels of α2-plasmin inhibitor (α2-PI) in Philadelphia chromosome-positive lymphoblastic leukemia patients who had suffered hemorrhagic episodes while on an IM dose of 400 mg per day. The authors suggested that other predisposing factors might play a role in the development of clinical bleeding as abnormal fibrinogenolysis is known to occur when plasma α2-PI levels fall below 60% of normal [11], and heterozygous patients with α2-PI deficiency exhibit hemorrhagic diathesis after events such as trauma, surgery, and dental extraction [12, 13]. In our patient, α2-PI was not checked at the time of the diagnosis of subdural hematoma. However, the α2-PI level was 108% (range, 80-120%) 2 months later when the patient was on an IM dose of 600 mg daily.
Recently, platelet aggregation dysfunction was reported in CML patients receiving dasatinib or IM [14]. Among the 15 patients on IM (5 at 400 mg/day, 4 at 600 mg/day, and 6 at 800 mg/day) not receiving aspirin, 10 had impaired arachidonic acid-induced platelet aggregation. Further investigations are needed in order to determine the mechanism of this platelet dysfunction. It leaves much to be desired that the platelet function tests were not performed at the time of the diagnosis of subdural hematoma in our case.
This report describes the occurrence of a subdural hematoma in a CML patient treated with 800 mg IM daily with a background of normal coagulation and no history of recent trauma, thrombocytopenia, or anti-coagulation medication. This suggests that IM may cause subdural hematomas by an as yet unknown mechanism. It is interesting to note that IM produces hemostatic abnormalities independent of thrombocytopenia. Further investigations are needed to establish the mechanism of subdural hematoma causation, and also to examine the reduction in α2-PI levels and platelet aggregation dysfunction described above. This case indicates that physicians should be alert to this atypical cause of headache in patients taking high-dose IM, including those who are in the advanced stage of CML.
References
2. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003; 348:994–1004. PMID: 12637609.
3. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001; 344:1031–1037. PMID: 11287972.

4. Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002; 99:1928–1937. PMID: 11877262.

5. Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:399–406. PMID: 10918008.

6. Druker BJ, Sawyers CL, Capdeville R, Ford JM, Baccarani M, Goldman JM. Chronic myelogenous leukemia. Hematology Am Soc Hematol Educ Program. 2001; 87–112. PMID: 11722980.

7. Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008; 111:4022–4028. PMID: 18256322.

8. Song KW, Rifkind J, Al-Beirouti B, et al. Subdural hematomas during CML therapy with imatinib mesylate. Leuk Lymphoma. 2004; 45:1633–1636. PMID: 15370217.

9. Radaelli F, Vener C, Ripamonti F, et al. Conjunctival hemorrhagic events associated with imatinib mesylate. Int J Hematol. 2007; 86:390–393. PMID: 18192104.

10. Matsue K, Aoki T, Odawara J, Kimura S, Yamakura M, Takeuchi M. Haemorrhagic complications associated with reduced alpha2-plasmin inhibitor during imatinib use in a patient with Philadelphia chromosome-positive acute lymphoblastic leukaemia. Leuk Res. 2009; 33:867–869. PMID: 18951628.
11. Okajima K, Kohno I, Tsuruta J, Okabe H, Takatsuki K, Binder BR. Direct evidence for systemic fibrinogenolysis in a patient with metastatic prostatic cancer. Thromb Res. 1992; 66:717–727. PMID: 1519230.

12. Kluft C, Vellenga E, Brommer EJ, Wijngaards G. A familial hemorrhagic diathesis in a Dutch family: an inherited deficiency of alpha 2-antiplasmin. Blood. 1982; 59:1169–1180. PMID: 6177359.

13. Favier R, Aoki N, de Moerloose P. Congenital alpha(2)-plasmin inhibitor deficiencies: a review. Br J Haematol. 2001; 114:4–10. PMID: 11472338.
14. Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009; 114:261–263. PMID: 19414863.

Fig. 1
Computed tomography and magnetic resonance imaging of the subdural hematoma. (A) Non-contrast axial CT scan shows a crescent-shaped, chronic CSF-isodense left subdural hematoma (arrow). There is mild effacement of the left lateral ventricle. (B) Axial FLAIR MR shows a chronic subdural hematoma with hyperintense signal (arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download