Abstract
The occurrence of granulocytic sarcoma as a pattern of relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is rare. In this paper, we report a rare case of acute myeloid leukemia (AML) relapsed as a granulocytic sarcoma of the donor type. The patient was diagnosed as having AML and underwent an allo-HSCT from his matched sibling donor. Fifty-seven months after allo-HSCT, he developed granulocytic sarcomas of duodenum, jejunum, and left sterno-cleido-mastoid muscle. The bone marrow was normal with 100% donor chimerism. A Y chromosome PCR was performed on the patient's duodenum specimen as well as bone marrow aspirate in order to check the patient-origin cells. The duodenal specimen was found to contain 41.2% SRY-positive cells (from the donor). Repeat endoscopy on day 2 of chemotherapy showed that the granulocytic sarcoma had shrunk dramatically. The patient died of sepsis during the nadir state 35 days after starting salvage chemotherapy.
Isolated extramedullary relapses of granulocytic sarcoma (GS) of acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) are reported to be rare and are usually followed by bone marrow relapses [1]. GS was first described by Burns in 1811 and subsequently termed "chloroma" by King in 1853. The association of chloroma with leukemia was first reported in 1904. The term "granulocytic sarcoma" was first used by Rappaport in 1967. Little is known concerning the mechanism of GS development, but the prognosis of patients with GS of AML is less favorable than that of AML patients with bone marrow relapse only [1-3]. Here, we present the case of a patient with AML who developed GS of donor origin defined to his duodenal mucosa, jejunum, and left sterno-cleido-mastoid (SCM) muscle 57 months after allo-HSCT and without any evidence of bone marrow recurrence.
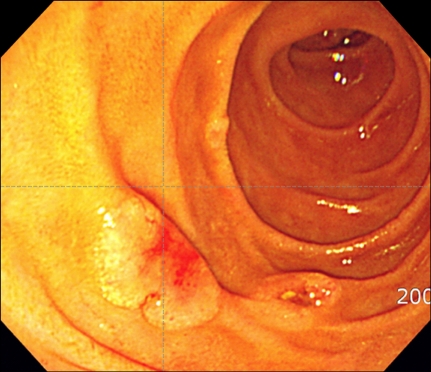
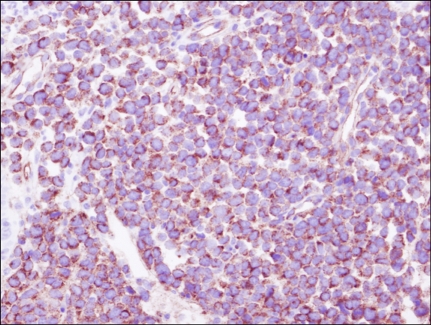
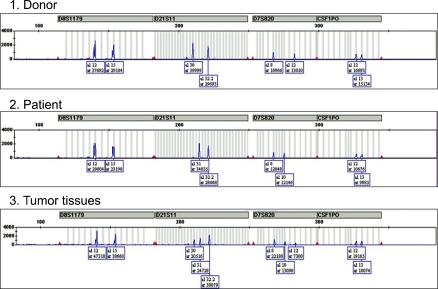
A 35-year-old man presented with fever, weight loss, and a sore throat, which had persisted for 2 months. He was diagnosed as having AML with positive expression of CD3, CD5, CD7, CD13, CD22, CD33, CD34, and cytoplasmic myeloperoxidase. Cytogenetic analysis of the leukemic cells showed a normal 46, XY karyotype. He was initially treated with idarubicin (12 mg/m2/d for 3 days) and cytarabine (200 mg/m2/d for 7 days). Follow-up bone marrow biopsy performed 28 d after the completion of chemotherapy showed complete remission of the acute leukemia. After 1 cycle of consolidation chemotherapy using the same regimen as used previously, the patient underwent an allo-HSCT from his HLA full-matched sibling (sister) donor. The conditioning regimen for the allo-HSCT was a combination of busulfan (4 mg/kg for 4 days) and cyclophosphamide (60 mg/kg for 2 days). Cyclosporine and methotrexate were administered for graft versus host disease (GVHD) prophylaxis after the allo-HSCT. Morphological complete remission and complete donor chimerism were confirmed by a bone marrow biopsy and chimerism study, respectively, performed at D+28 after the HSCT. Ten months after the HSCT, grade III GVHD developed in the patient's liver; however, this was successfully treated with steroids and cyclosporin. Fifty-seven months after the HSCT, the patient developed gastrointestinal (GI) symptoms of nausea, vomiting, and epigastric pain. Endoscopic examination revealed a mass lesion growing into the lumen of the second portion of the duodenum (Fig. 1), and subsequent biopsy confirmed the duodenal lesion as a GS (Fig. 2). Multiple jejunal mass involvement was observed on a computed tomography (CT) scan. The bone marrow was, nevertheless, free of acute leukemic blast cells and a chimerism study again showed complete donor chimerism. The chimerism study performed on a duodenal biopsy specimen by multiplex short tandem repeat amplification revealed that 41.2% of the cells were of donor origin (Fig. 3). During the period of evaluation of the duodenal lesion and bone marrow, the patient developed a mass lesion in his left SCM muscle. The specimen obtained from the neck mass by needle aspiration biopsy also showed aggregations of acute leukemic cells. The patient was administered second-line induction chemotherapy comprising mitoxantrone (10 mg/m2/d for 3 days), etoposide (100 mg/m2/d for 4 days), and cytarabine (2,000 mg/m2/d for 5 days). Repeated endoscopy on day 2 of the chemotherapy showed that the GS had shrunk dramatically. However, pneumonia and sepsis developed during a neutropenic period after chemotherapy and the patient died of septic shock.
The incidence of GS in the course of AML has been reported to range from 3% to 4.7% [4]. Cases of isolated extramedullary relapse of myeloid malignancies following an allo-HSCT are much rarer [5]. There appears to be a tendency for acute leukemias to relapse in soft tissue and to continue to relapse in soft tissues more often than in bone. Frequent sites of involvement are the skin, head and neck area, breast, bone, testis, and serosa; however, sites such as the GI tract, pancreas, heart, and appendix are also reported to be involved, although rarely [6-10]. GI tract involvement is considerably rarer than that of the other sites with only few cases reported [4, 7, 11].
In a retrospective survey conducted by European transplantation centers, only 0.65% of patients with AML undergoing allo-HSCT developed isolated extramedullary recurrences, and 95% of these were followed by a bone marrow relapse within 1-12 months of diagnosis [1]. Since our patient developed involvement at another site during the evaluation period of the duodenal GS, subsequent development of bone marrow involvement was considered highly probable. The risk period for first extramedullary relapse in acute lymphoblastic lymphoma appears to be 2 years after transplantation; however, in myeloid leukemias, one-third of the cases occurred after 2 years [7]. In our case, GS developed 57 months after transplantation.
Donor cell-origin leukemic recurrence after allo-HSCT has been reported in several cases; however, donor cell-origin GS is extremely rare [12-14]. A report from Italy described a patient who suffered 2 recurrences of GS of patient cell origin and a third recurrence with leukemia mixed with cells of donor origin [13]. In the patient from Italy, subsequent bone marrow recurrence with patient-origin leukemia also developed. In our patient, a chimerism study revealed that 41.2% of cells were of donor origin. It is reasonable to consider that the GS developed from donor cells and mixed with the intestinal epithelium of the patient during endoscopic biopsy. To the best of our knowledge, this is the second report of GS of donor cell origin and the first report of GS of donor origin involving the GI tract.
Extramedullary relapse following allo-HSCT is a very poor prognostic sign [4, 5, 15]. Unless the initial treatment is adequate, a single relapse focus is almost invariably followed by other extramedullary relapses, usually within months [7]. Once a single focus is clinically observed, it is likely that other sites will become evident if aggressive systemic treatment is not undertaken expeditiously, even when the marrow shows no leukemic cells [7]. When more than 1 site is evident, durable remission and disease-free survival have rarely been reported. Extramedullary relapse has typically not been successfully treated with local therapy alone, even when the bone marrow shows donor cells and no leukemia [7]. In such instances, local radiotherapy (RT) could be a treatment option. From the European data, 3 out of 6 patients treated with local RT without chemotherapy showed long-term survival; however, local RT to the GI tract is limited by its toxicity [1]. In our case, the disease progression was extremely fast, and within a couple of days further extramedullary site involvement in his left SCM muscle was observed. Although the GS shrunk markedly after combination chemotherapy, the patient failed to recover from sepsis. Management of extramedullary recurrences in the post-transplantation setting is extremely difficult because of the cumulative toxicities of previous high-dose chemotherapy. Investigation of an optimal treatment strategy is therefore needed in order to manage highly aggressive GS in fragile patients after HSCT.
References
1. Bèkássy AN, Hermans J, Gorin NC, Gratwohl A. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Bone Marrow Transplant. 1996; 17:801–808. PMID: 8733701.
2. Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995; 13:1800–1816. PMID: 7602369.

3. Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003; 32:835–842. PMID: 14520431.

4. Breccia M, Mandelli F, Petti MC, et al. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res. 2004; 28:1165–1169. PMID: 15380340.

5. Koc Y, Miller KB, Schenkein DP, Daoust P, Sprague K, Berkman E. Extramedullary tumors of myeloid blasts in adults as a pattern of relapse following allogeneic bone marrow transplantation. Cancer. 1999; 85:608–615. PMID: 10091734.

6. Kara IO, Sahin B, Paydas S, Kara B. Granulocytic sarcoma of the heart: Extramedullary relapse of acute myeloblastic leukemia after allogeneic stem cell transplantation successfully treated by chemotherapy alone. Leuk Lymphoma. 2005; 46:1081–1084. PMID: 16019562.

7. Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006; 47:1754–1767. PMID: 17064985.

8. Toubai T, Kondo Y, Ogawa T, et al. A case of leukemia of the appendix presenting as acute appendicitis. Acta Haematol. 2003; 109:199–201. PMID: 12853694.

9. Servin-Abad L, Caldera H, Cardenas R, Casillas J. Granulocytic sarcoma of the pancreas. A report of one case and review of the literature. Acta Haematol. 2003; 110:188–192. PMID: 14663163.
10. Park J, Park SY, Cho HI, Lee D. Isolated extramedullary relapse in the pleural fluid of a patient with acute myeloid leukemia following allogeneic BMT. Bone Marrow Transplant. 2002; 30:57–59. PMID: 12105780.

11. Kitagawa Y, Sameshima Y, Shiozaki H, et al. Isolated granulocytic sarcoma of the small intestine successfully treated with chemotherapy and bone marrow transplantation. Int J Hematol. 2008; 87:410–413. PMID: 18365139.

12. Daly AS, Kamel-Reid S, Lipton JH, et al. Acute leukemia of donor origin arising after stem cell transplantation for acute promyelocytic leukemia. Leuk Res. 2004; 28:1107–1111. PMID: 15289025.

13. Pelosini M, Benedetti E, Galimberti S, et al. Granulocytic sarcoma and subsequent acute leukemia recurrence with different biologic characteristics 5 years after allogeneic bone marrow transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2006; 37:897–898. PMID: 16547489.

14. Ataergin S, Arpaci F, Cetin T, et al. Donor cell leukemia in a patient developing 11 months after an allogeneic bone marrow transplantation for chronic myeloid leukemia. Am J Hematol. 2006; 81:370–373. PMID: 16628734.

15. Kikushige Y, Takase K, Sata K, et al. Repeated relapses of acute myelogenous leukemia in the isolated extramedullary sites following allogeneic bone marrow transplantations. Intern Med. 2007; 46:1011–1014. PMID: 17603242.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download