INTRODUCTION
AML is a clinically and genetically heterogeneous disease. Three cytogenetically defined risk groups (favorable, intermediate, and adverse) are used to design risk-adapted treatment protocols for patients with AML. However, about 35-50% of the successfully karyotyped patients lack clonal chromosomal aberrations [
1], and the prognostic implications have not been clearly established for cytogenetically normal (CN)-AML [
2]. Therefore, it is important to develop a molecular-level genetic approach for discriminating between prognostically different subsets of CN-AML.
In the recent years, CN-AML has been identified as a heterogeneous disease with mutations of the nucleophosmin (
NPM1) and
fms-like tyrosine kinase 3 (
FLT3) genes.
NPM1, encoded by the
NPM1 gene on chromosome 5q35, is a multifunctional nucleocytoplasmic shuttling protein localized primarily in the nucleolus but shuttles rapidly between the nucleus and the cytoplasm. The protein appears to be important for various cellular processes. NPM may assist in ribosomal protein assembly [
3] and maintain genomic stability through its participation in DNA repair [
4]. It also plays a crucial role in cell cycle regulation and apoptosis via its interactions with tumor suppressor p53 and alternate reading frame protein [
5,
6].
The
NPM1 gene frequently acts as a target of chromosomal translocations and causes the cytoplasmic dislocation of proteins in various types of leukemia and lymphoma, indicating its role in malignant transformation. Recent studies have demonstrated that aberrant cytoplasmic localization of NPM (NPMc+) in leukemic blasts is associated with mutations at exon-12 of the
NPM1 gene [
7-
9].
NPM1 exon-12 mutations can encode mutant proteins with a novel nuclear export signal (NES) motif inserted at the C-terminus and disruption of the nucleolar localization signal due to mutations of tryptophan residues 288 and 290 [
7,
8]. Such mutations are classified according to the type of NES motif inserted into the mutant protein. In adult NPMc+ AML, mutation 'A' (tandem duplication of TCTG) accounts for approximately 80% of all the NPMc+ cases [
9]. Mutations at
NPM1 exon-12 and the resultant shift of
NPM1 into the cytoplasm are found in approximately 35% of the adults with AML. One of the most frequent mutations seen in CN-AML is
NPM1 mutation (
NPM1mut), found in 45.7-64% of the patients. Besides the characteristic biological features such as increased frequency of monocytic leukemia and distinctive gene expression profiles, patients with CN-AML carrying isolated
NPM1mut show better clinical outcomes in terms of the responsiveness to chemotherapy or disease-free survival (DFS) than those with the wild-type
NPM1 (
NPM1wt) gene [
9-
12].
FLT3 belongs to the class III receptor tyrosine kinase family. It is expressed in early hematopoietic progenitors and its dimerization by the
FLT3 ligand induces growth control signals in normal hematopoiesis. The
FLT3 gene maps to chromosome band 13q12 [
13], and an internal tandem duplication (ITD) of the gene (
FLT3-ITD) has been detected in 20-30% of the young adults with AML [
14]. The duplication involves a segment of the juxtamembrane domain-coding sequence, which frequently involves exon-14 and rarely intron-14 or exon-15, and is always in-frame [
14]. In support of the potential role of this mutation in leukemogenesis,
FLT3-ITD from patients with AML has been shown to induce autonomous proliferation in cytokine-dependent cell lines. In contrast to
NPM1mut, patients with isolated
FLT3-ITD mutation (
FLT3-ITD+) show poor clinical outcomes, similar to those with poor-risk cytogenetics [
9,
12,
14].
Several studies have attempted to identify the interactions between these frequent mutations and their prognostic implications for patients with CN-AML. On the basis of these reports, the recently updated National Comprehensive Cancer Network (NCCN) Guidelines [
15] recommend that isolated
NPM1mut and
FLT3-ITD+ CN-AML should be regarded as better-risk and poor-risk cytogenetics, respectively; hence, transplantation as a postremission therapy should be reserved until relapse in patients with CN-AML carrying isolated
NPM1mut. In contrast, patients with
FLT3-ITD+ CN-AML should be enrolled in clinical trials as the standard therapy on account of their poor prognosis. Nonetheless, controversy exists as to the prognostic significance of these two mutations in patients with CN-AML [
9,
16,
17]. Therefore, we examined the incidence and interactions of
NPM1mut and
FLT3-ITD+ in such patients and assessed the validity of the newly proposed risk-adapted treatment strategies based on these mutations.
Go to :

MATERIALS AND METHODS
1. Patients and treatment protocol
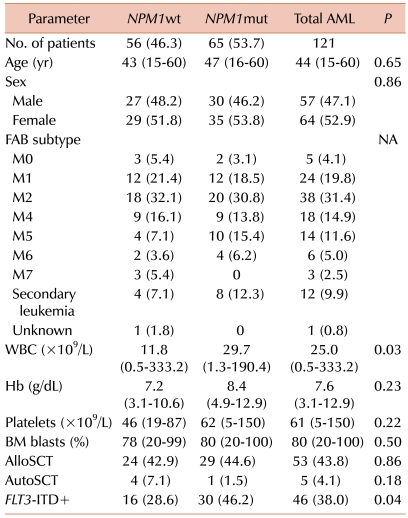
Diagnostic bone marrow (BM) samples from 121 adult patients with CN-AML (age≤60 years) who had received at least one cycle of intensive induction chemotherapy were analyzed retrospectively for the presence of
NPM1mut and
FLT3-ITD+. To establish CN-AML, 20 or more metaphase cells from the samples had to test positive for normal karyotypes [
18]. Approval was obtained from the institutional review board for this procedure. All patients gave written informed consent for both the treatment and the cryopreservation of BM.
All enrolled patients received intensive remission induction therapy consisting of 3 days of idarubicin (IDA) at 12 mg/m2/day and 7 days of cytarabine at 100 mg/m2/day or N4-behenoyl-1-D-arabinofuranosylcytosine (BH-AC; 300 mg/m2/day for patients younger than 40 years, 200 mg/m2/day for patients older than 40 years). If the patients failed to achieve complete remission (CR) after the first round of induction chemotherapy, they received reinduction chemotherapy using the same regimen. Patients achieving CR received three courses of high-dose cytarabine chemotherapy (HDAC; 3 g/m2 every 12 h/day on days 1, 3, and 5) for consolidation. These patients were allowed to proceed to allogeneic (alloSCT) or autologous stem cell transplantation (autoSCT) after one or two courses of HDAC consolidation therapy.
2. Mutational analysis
Genomic DNA was extracted from the diagnostic BM samples, such as cryopreserved mononuclear cells, by using a DNA blood minikit (QIAamp; Qiagen, Valencia, CA, USA) according to the manufacturer's protocol.
For NPM1mut analysis, NPM1 exon-12 was amplified by genomic PCR using primers 5'-TCTGAGTATAAATTTTCTTGGAGTCA-3' (sense) and 5'-ACCAAGCAAAGGGTGGAGTT-3' (antisense). The reaction mixture contained 1.25 pmol of each primer, 50 ng of genomic DNA, 250 µM dNTPs, and 0.5 U f-taq polymerase (Solgent, Daejeon, Korea) in the buffer provided by the manufacturer. Amplification was performed in a thermal cycler (PTC 200; MJ Research, Inc., Waltham, MA, USA), and the PCR fragments were purified (GENEALL PCR Purification Kit; General Biosystem, Seoul, Korea). The sequencing reactions were analyzed by using a sequencer (ABI 3100) and cycle sequencing kit (BigDye Terminator; Applied Biosystems, Foster City, CA, USA).
For FLT3-ITD+ analysis, wild-type FLT3 exon-11 and exon-12 were amplified by genomic PCR using primers 5'-CAATTTAGGTATGAAAGCC-3' (sense) and 5'-CTTTCA GCATTTTGACGGCAACC-3' (antisense). The reaction mixture contained 2.5 mM dNTPs, 2.5 mM MgCl2, 0.5 µM of each primer, and 0.5 U f-taq polymerase in a total volume of 20 µL. The samples were amplified by initial denaturation at 95℃ for 5 min, followed by 35 cycles of 94℃ for 30 sec, 53℃ for 1 min, and 72℃ for 2 min, and final extension at 72℃ for 10 min. The PCR products (10 µL) were resolved on 6% polyacrylamide gels, stained with ethidium bromide, and photographed under ultraviolet light.
3. Statistical analysis
The response to initial therapy was evaluated after induction or after salvage chemotherapy. The definition of CR followed the recommended criteria [
19]. Relapse was defined as the reappearance of blasts post-CR in the peripheral blood or BM. Relapse-free survival (RFS) endpoints, measured from the date of documented CR, included relapse, patient death from any cause, and alive in CR at last follow-up (censored). The overall survival (OS) endpoints, measured from the date of diagnosis, were death from any cause and alive at last follow-up (censored) [
19]. RFS before transplantation and OS before transplantation were also assessed to eliminate confounding bias and were defined as the time without relapse, death, or transplantation from the date of CR and the time from diagnosis to death or transplantation, respectively.
For between-group comparisons, Fisher's exact test (categorical data) and the Mann-hitney U test (continuous data) were used. Categorical data were compared among three groups defined by the NPM1 and FLT3-ITD mutation status using one-way analysis of variance (ANOVA) and the post hoc Tukey's honestly significant difference (HSD) test. Continuous variables were compared among the three groups by using the Kruskal-Wallis test. RFS and OS were analyzed by means of Kaplan-Meier survival curve estimates and logrank tests to compare differences in the distribution of survival for the three groups. Multivariate analysis using forward conditional selection of variables was performed with the Cox's proportional-hazards model to analyze the influence of high WBC count (>50×109/µL versus ≤50×109/µL), secondary AML (versus de novo AML), alloSCT, autoSCT, and the NPM1 and FLT3-ITD mutation status or the interaction of these mutations. A P-value of less than 0.05 was considered statistically significant. All statistical computations were performed by using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA).
Go to :

DISCUSSION
We evaluated the prevalence and prognostic impact of
NPM1mut and
FLT3-ITD+ and their interactions in adult patients with CN-AML. The incidence of
NPM1mut and
FLT3-ITD+ was 53.7% and 38%, respectively, suggesting that they are frequent molecular events in such patients. The incidence of
FLT3-ITD+ was higher in the
NPM1mut group than in the
NPM1wt group. Previous studies have reported a considerable correlation between these two mutations, suggesting that they are secondary events from a primary process that predisposes myeloid stem and progenitor cell errors in DNA replication [
10,
11]. Others have demonstrated that the
NPM1mut/allele ratio is higher than the
FLT3-ITD+/wild-type ratio, suggesting that
NPM1mut occurs prior to
FLT3-ITD+ in cases with both mutations [
9].
According to previous studies, patients with CN-AML carrying
NPM1mut show a higher rate of CR, suggesting that these patients are more sensitive to chemotherapeutic agents [
11,
12,
20]. These studies assumed that NPMc+ may interact with and sequester nuclear factor kappaB (NF-κB), contributing to the maintenance and survival of malignant clones and an impaired response to chemotherapy in the cytoplasm, leading to its inactivation and reduced DNA binding [
20]. However, in other studies, patients younger than 60 years and pediatric patients did not show a significantly different CR rate between
NPM1wt and
NPM1mut [
9,
16,
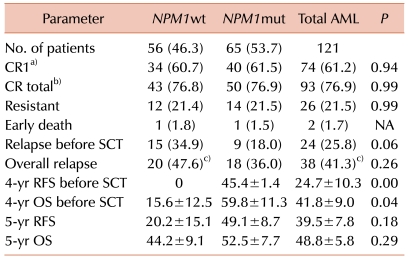
17]. In the present study, there were no significant differences in the CR rate between
NPM1mut and
NPM1wt. Although the patients with
NPM1mut/
FLT3-ITD- CN-AML showed a slightly higher CR rate than the other groups, the differences were not statistically significant.
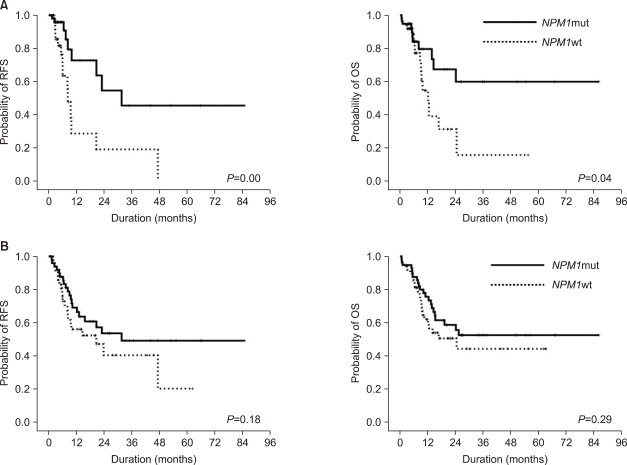
On the other hand, we demonstrated that the
NPM1mut group had better RFS and OS before SCT than the
NPM1wt group, although the differences were not statistically significant. A recent study has demonstrated that non-A type
NPM1mut individuals show different clinical outcomes compared with those having the type A mutation [
21]. In the present study, however, all the
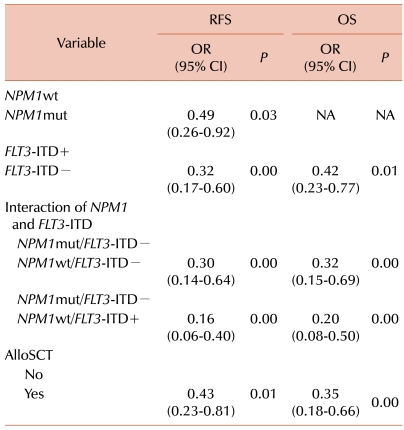
NPM1mut individuals possessed type A mutations. In the multivariate analysis to evaluate other factors influencing the overall RFS and OS,
FLT3-ITD+ and alloSCT treatment were identified as independent prognostic factors (besides the
NPM1mut status).
For CN-AML, the proper risk-stratified postremission therapy has not yet been determined. Previous studies on young adult patients with CN-AML have reported a 4-year DFS of 48.5% with alloSCT [
22] and 5-year DFS of 28-41% with repeated courses of HDAC consolidation [
23,
24]. Hence, transplant-based options generally afford a lesser risk of relapse and higher DFS as consolidation for the patients. However, this benefit is accompanied by a treatment-related mortality of 15-25% [
25]. To balance the treatment-related toxicity and risk of relapse, it is crucial to define distinct clinical and molecular subtypes that influence the prognosis
of CN-AML.
It was previously reported that event-free survival (EFS) and DFS are better in patients with NPMc+/
FLT3-ITD- than in those with other CN-AML subtypes [
9,
12]. Some studies have also shown that the 5-year EFS and OS of patients with NPMc+/
FLT3-ITD- are comparable to those with favorable cytogenetics [
16,
17], suggesting that this group of patients could be considered a favorable group. Others have reported that the RFS and OS are significantly better in the NPMc+/
FLT3-ITD- group than the other groups, and suggested that alloSCT has no effect on the RFS and OS in this subset of CN-AML [
11]. Therefore, the NCCN guidelines for AML consider patients with isolated
NPM1mut as a favorable-risk group and recommended multiple cycles of HDAC as a reasonable option. Further, patients carrying isolated
FLT3-ITD+ constitute a poor-risk cytogenetic group that should be considered for clinical trials or early alloSCT [
15]. However, other studies have reported variable results about the clinical outcomes according to the presence of these mutations; the
FLT3-ITD+ status was prognostically significant only in the patients with
NPM1wt [
18,
19].
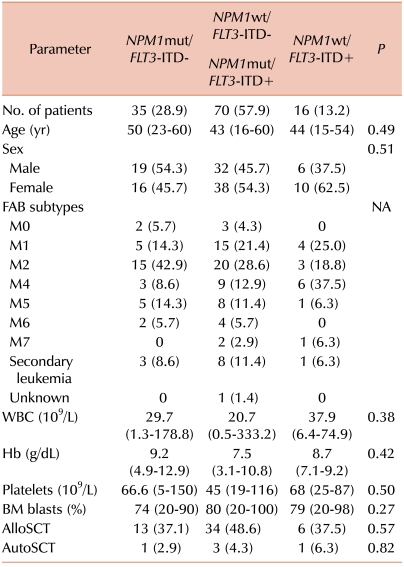
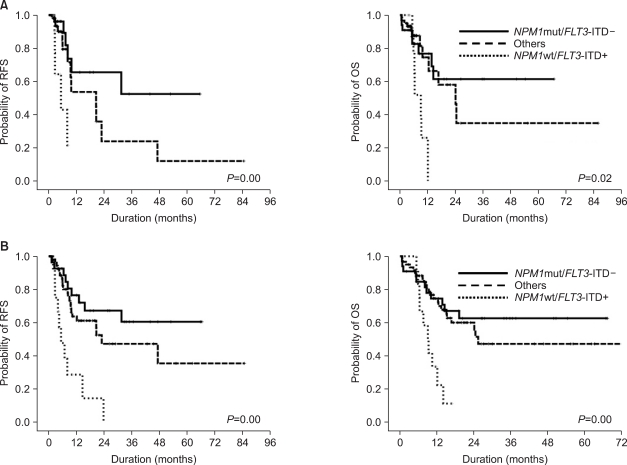
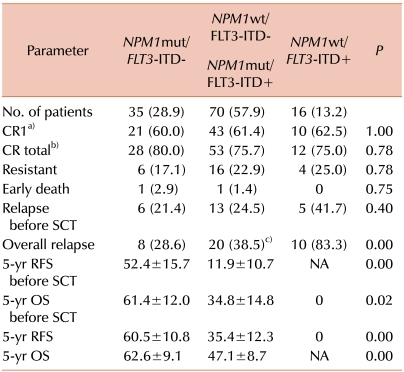
In our three-group analysis, the isolated
NPM1mut group had a significantly better overall relapse rate and the highest rate of 5-year RFS and OS, regardless of SCT. Further, in this group, alloSCT significantly increased the 5-year RFS rate. For patients carrying favorable cytogenetics such as t(8;21) or inv (16), multiple courses of HDAC are known to lead to 50-60% of 4-year DFS [
24,
26]. In the present study, the 5-year RFS was 42.7% for the patients treated only with HDAC consolidation in the isolated
NPM1mut group, lower than that among patients with favorable cytogenetics. The patients who underwent alloSCT, however, showed a significantly better 5-year RFS rate (87.5%) than the chemotherapy-alone patients in the isolated
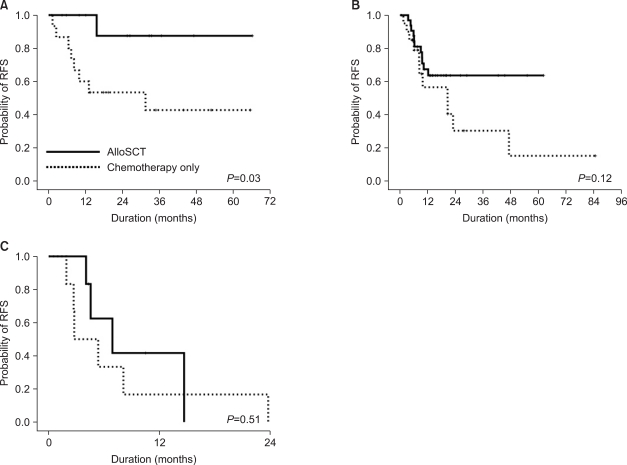
NPM1mut group. Therefore, the role of alloSCT in patients with
NPM1mut/
FLT3-ITD- CN-AML should be reconsidered.
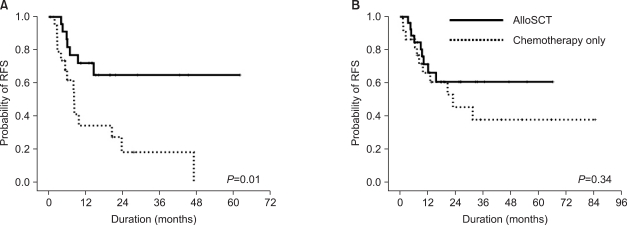
In contrast, the isolated FLT3-ITD+ group showed poor clinical outcomes in terms of the overall relapse rate, RFS, and OS, regardless of SCT. All patients relapsed or died within 2 years after diagnosis, and alloSCT did not change their RFS.
In the NPM1wt/FLT3-ITD- or NPM1mut/FLT3-ITD+ group, the 5-year RFS increased remarkably (but not significantly) with alloSCT compared with the chemotherapyalone patients, who showed a remarkably lower rate of 5-year RFS (15.1%) than the patients who underwent alloSCT (63.6%). Therefore, alloSCT seems to be a reasonable option for this subset of patients.
In summary, adult patients (≤60 years) with CN-AML carrying isolated NPM1mut and FLT3-ITD+ show different clinical outcomes than those bearing both mutated or wild-type NPM1 and FLT3-ITD. Furthermore, isolated NPM1mut is associated with favorable clinical outcomes in patients with CN-AML; however, the efficacy of alloSCT as a treatment option for this group of patients remains to be determined.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download