Abstract
Primary cardiac lymphoma (PCL) is a rare disease entity with only a few reported cases in Korea. In this paper, we report a case of PCL in a 59-year-old man presenting with chest pain. Diffuse large B-cell lymphoma was diagnosed through a cardiac catheterization-assisted percutaneous endomyocardial biopsy, and there was no evidence of extracardiac involvement of the lymphoma.The patient had a complete clinical response after systemic chemotherapy with a rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) regimen and additional post-chemotherapeutic radiation therapy. The patient experienced a long-term disease-free survival of over 4 years. However, he received coronary artery bypass graft surgery due to an acute myocardial infarction that occurred 3 years after the completion of the radiation therapy. Although the addition of radiation therapy to the treatment is thought to decrease the risk of relapse in patients with PCL, a careful and thorough consideration of the potential complications of radiation therapy, particularly with respect to cardiac complications, should be considered.
Primary cardiac lymphoma (PCL), which makes up about 2% of all primary cardiac tumors, is extremely rare in immunocompetent hosts [1]. The median age of patients with PCL is 64 years, and it occurs 3 times more frequently in women than in men [2]. PCL mainly involves the right atrium and the right ventricle. However, any area of the heart can be involved. The symptoms at initial presentation are nonspecific and examples of symptoms include obstruction of blood flow, tumor invasion to adjacent organs, symptoms indicating tumor emboli, treatment-refractory heart failure, dyspnea, arrhythmia, superior vena cava syndrome, and stroke. Imaging modalities such as echocardiography (ECG), computed tomography (CT), and magnetic resonance imaging (MRI) are helpful, and pathologic findings by tissue biopsy or surgical resection are essential for a confirmative diagnosis. In this paper, we present a case of a 59-year-old man who presented with chest pain. A huge mass in his right atrium was detected, and diffuse large B-cell lymphoma was diagnosed through a percutaneous endomyocardial biopsy. He experienced a disease-free state of over 4 years of outpatient follow-up, after he received the systemic chemotherapy regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) and additional post-chemotherapeutic radiation therapy.
A 59-year-old man without a medical history visited a local clinic due to a 1-month history of chest pain. An ECG was performed, and a mass-like lesion was detected in the right atrium. He was immediately referred to the emergency room of our hospital. He complained of intermittent chest pain and dyspnea on exertion. He did not have any medical history or risk factors for cardiac disease. He also denied a family history of cardiac disease or malignancy.
The patient's vital signs were stable. His blood pressure was 121/79 mmHg, pulse rate was 76/min, respiratory rate was 18/min, and body temperature was 36.4℃. His heart sounds exhibited a slightly accelerated regular rhythm without a murmur. A fixed S2 split of his heart sounds was heard. His lung sounds were vesicular without crackling or wheezing. His bowel sounds were normoactive, and there was no tenderness or rebound tenderness in the abdomen. There was no organomegaly such as hepatomegaly or splenomegaly. No peripheral lymphadenopathy or edema was detected.
The laboratory findings of the patient's peripheral blood samples were as follows: white blood cell count, 8,500/µL; hemoglobin, 14.4 g/dL; and platelets, 352,000/µL. The results of serology were as follows: HBs Ag/Ab (-/+); anti-HCV (-); and anti-HIV (-). Lactate dehydrogenase (LDH) was elevated to 248 IU/L. A simple chest X-ray showed nonspecific findings except for mild cardiomegaly.
An initial ECG showed a first-degree atrioventricular block, a right axis deviation, and a suspicious right ventricular hypertrophy. A transthoracic ECG showed a 6×4 cm-non-movable ovoid mass with a broad fundus in the atrial and membranous septum of the right atrium. This mass nearly obstructed the inflow to the right ventricle, which resulted in reduced diastolic volume of the right ventricle. The ejection fraction of the left ventricle was 69%, and a moderate amount of pericardial effusion was present.
A chest CT showed that the poorly enhanced tumor pressed on both the atria and part of the left ventricle and was accompanied by pericardial effusion. There were no abnormal findings on abdomen CT. A cardiac MRI showed a mass that invaded the left atrium and extended to the pericardial space and intra-atrial cavity (Fig. 1A). This mass encased the root of the aorta, the proximal part of the right coronary artery, and the left main coronary artery. It showed an iso-tense signal intensity on a T2-weighted image, a diffuse enhancement pattern on a contrast-weighted image, and an iso-tense or slightly higher signal intensity compared to that of cardiac muscle on a T1-weighted image. There were no findings suggestive of myocardial infarction.
A cardiac catheter-guided endomyocardial biopsy was performed at 8 sites in the heart. The pathologic findings were consistent with diffuse large B-cell lymphoma, and the specimen was CD20-positive (Fig. 1B, C). The percentage area of Ki-67 labeling was 60%. Immunoreactivity for bcl-2 and bcl-6 was also positive. Tumor invasion of the malignant lymphoma at any area except the heart was not detected in a bone marrow examination or in the imaging studies.
The patient received systemic chemotherapy of a rituximab, cyclophosphamide, adriamycin, vincristine, and prednisolone (R-CHOP) regimen every 3 weeks. The size of the mass was remarkably reduced after 1 cycle of R-CHOP, and further reduced after 6 cycles of chemotherapy (Fig. 1D). The best response of R-CHOP was a partial response (PR).
The patient received radiation therapy to the cardiac mass while in the PR state, and the total dose of radiation was 39.6 Gy in 22 fractions. However, the size of the cardiac mass did not change despite the radiation therapy, and the disease state continued to be PR after the completion of the radiotherapy. The patient visited the emergency room due to chest pain 3 years after the completion of the radiation therapy. The cardiac imaging showed no change in the size of the cardiac tumor, whereas the findings of the coronary angiography showed total occlusion of the proximal part of the left anterior descending (LAD) coronary artery. He received coronary artery bypass graft surgery due to an acute myocardial infarction of the LAD territory.
The disease state of the patient has been complete clinical remission over the 4 years since the initial diagnosis of PCL.
To our knowledge, this is the first report of a case with long-term disease-free survival in a Korean patient with PCL treated with systemic chemotherapy combined with post-chemotherapeutic radiation therapy. A summary of cases of PCL in Korea is available elsewhere [3].
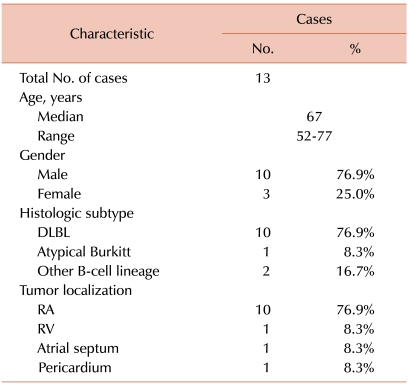
In order to compare this case with previous reports of patients with PCL, we searched case reports from the database of the National Library of Medicine of the United States (http://www.ncbi.nlm.nih.gov/pubmed/) according to the following criteria: (1) published from Jan 1995 to Dec 2009, (2) treated with systemic chemotherapy (except adjuvant chemotherapy), (3) long-term follow-up data (≥1 years) was available, and (4) written in English. We found 12 cases that met our criteria. The analysis of the previous reports showed that the predominant gender was male (75.0%). The most common histologic subtype was diffuse large B-cell lymphoma, and the most frequent site of the tumor was the right atrium (Table 1). The most frequent location of PCL reported in our results was different from that of myxoma, which is the most common primary cardiac tumor.
The clinical presentation of PCL is nonspecific, and hence, an imaging study that is conducted because of a suspicion for PCL is essential to the initial diagnosis. There are a few primary cardiac malignancies that mimic PCL, such as angiosarcoma and rhabdomyosarcoma. Angiosarcoma is also often located within the right atrium.
Angiosarcoma is usually located in the central region and shows an iso-tense signal intensity, which is consistent with central necrosis on T1-weighted images, whereas PCL shows a nodular mass without intratumoral necrosis of an iso-tense signal intensity on T1-weighted images. Rhabdomyosarcoma that originates from the myocardium has no favorable location and frequently invades the valvular structure. An imaging study of rhabdomyosarcoma shows a huge mass with central necrosis and a characteristic inhomogeneous signal intensity on T1- and T2-weighted images [4]. Despite the few differential considerations of primary cardiac malignancies described above, imaging modalities cannot be confirmative to diagnosis. For example, PCL may have less enhanced central regions, which is consistent with necrosis [5]. Thus, a final confirmative diagnosis should be proven through pathology.
Although fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)-CT is performed for the staging in many cases, the diagnostic sensitivity of a cardiac lesion is very low because the baseline 18FDG uptake of the heart is very high [6]. Therefore, CT and MRI, rather than PET-CT, were performed in the evaluation of our patient.
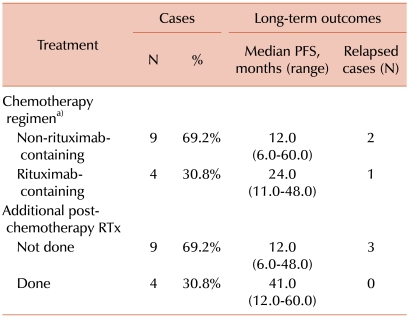
The standard treatment option of PCL is not well established, and various treatment modalities, such as surgical resection, systemic chemotherapy, radiation therapy, and combined treatments, have been attempted. The analysis of the 13 case reports, including the current case report, showed that all patients received anthracycline-containing combination chemotherapy with or without additional post-chemotherapeutic radiation therapy. Although the number of patients enrolled in our analysis was too small to elicit statistical significance, our analysis of the case reports of PCL showed that the patients who were treated with a combined modality of systemic chemotherapy and additional post-chemotherapeutic radiation therapy had a better long-term progression-free survival (PFS) compared to those treated with systemic chemotherapy alone (median PFS, 41.0 months vs. 12.0 months). Furthermore, no recurrence of lymphoma was noted in the patients who received both systemic chemotherapy and additional post-chemotherapeutic radiation therapy.
The patients who received rituximab-containing regimens had a longer PFS compared to those who received non-rituximab-containing regimens (median PFS, 24.0 months vs. 12.0 months). However, 1 case of recurrence was noted among the patients who had received rituximab-containing regimens (Table 2).
Our analysis of this small case series of PCL patients showed that the combined treatment of chemotherapy and post-chemotherapeutic radiation therapy decreased the risk of relapse. The cardiac mass of the patient in our case showed a residual lesion that was unchanged for a long time after the completion of the radiation therapy, which suggested the probability that non-tumorous fibrous tissue replaced the original tumor subsequent to the chemotherapy-induced tumor necrosis.
Although cases of cardiac perforation as an initial presentation have been reported [7, 8], a post-chemotherapeutic complication of a cardiac perforation has not been reported yet. However, radiation therapy to the heart has been known to cause cardiac complications. A previous report showed that myocardial infarction developed in 4.5% of non-small cell lung cancer patients who had received previous radiation therapy [9], and another report of 415 patients with Hodgkin's disease [10] showed that coronary artery disease developed in 42 patients (10.4%), a median of 9 years after radiation therapy. Other cardiac diseases such as valvular heart disease, pericardial disease, and cardiomyopathy have also been reported.
Although the patient described in this case report had no risk factors for coronary artery disease, an acute myocardial infarction developed 3 years after the completion of radiation therapy, and was successfully treated by coronary artery bypass graft surgery. Although the addition of radiation therapy is thought to decrease the risk of relapse in the treatment of patients with PCL, a careful and thorough consideration of the potential complications of radiation therapy should be considered.
References
1. Burke A, Virmani R. Rosai J, Sobin LH, editors. Atlas of tumor pathology: tumors of the heart and great vessels. Atlas of Tumor Pathology. Fascicle 16. 3rd series. 1996. Washington, DC: Armed Forces Institute of Pathology;p. 80–86.
2. Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997; 80:1497–1506. PMID: 9338475.
3. Jang JY, Lee JJ, Han JJ, et al. A case of primary cardiac lymphoma with superior vena cava syndrome and sinus bradycardia. Korean J Med. 2009; 77(Suppl 2):S445–S450.
4. Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009; 64:1214–1230. PMID: 19913133.

5. Dorsay TA, Ho VB, Rovira MJ, Armstrong MA, Brissette MD. Primary cardiac lymphoma: CT and MR findings. J Comput Assist Tomogr. 1993; 17:978–981. PMID: 8227590.
6. Kaderli AA, Baran I, Aydin O, et al. Diffuse involvement of the heart and great vessels in primary cardiac lymphoma. Eur J Echocardiogr. 2010; 11:74–76. PMID: 19759028.

7. Menotti A, Imperadore F, Pelosi G, Disertori M. Heart rupture at the right atrial level as the first manifestation of malignant lymphoma. Cardiologia. 1996; 41:65–67. PMID: 8697472.
8. Molajo AO, McWilliam L, Ward C, Rahman A. Cardiac lymphoma: an unusual case of myocardial perforation-clinical, echocardiographic, haemodynamic and pathological features. Eur Heart J. 1987; 8:549–552. PMID: 3609049.

9. Lee CB, Stinchcombe TE, Moore DT, et al. Late complications of high-dose (>/=66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009; 4:74–79. PMID: 19096310.
10. Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003; 290:2831–2837. PMID: 14657067.

Fig. 1
(A) T2-weighted MRI image showing a cardiac mass (arrow) with similar signal intensity compared to the myocardium, and which had invaded the pericardial space and the right atrial wall with an intracardiac extension. (B) Biopsy specimen showing large, atypical lymphoid cells with irregularly shaped nuclei containing clumped chromatin and prominent nucleoli (hematoxylin and eosin stain, ×400). (C) CD20 immunohistochemistry of a biopsy specimen showing diffuse membranous staining. (D) A magnetic resonance image performed after 6 cycles of R-CHOP showing a partial response of the cardiac mass (arrow).
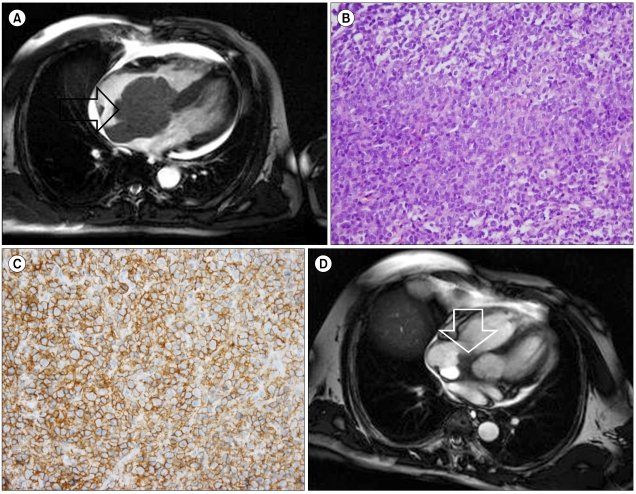
Table 1
Baseline characteristics of the long-term follow-up cases (≥1 year) with primary cardiac lymphoma treated with systemic chemotherapy (Data are from 13 cases, including the present case).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download