Abstract
Wernicke's encephalopathy is caused by thiamine deficiency, and is characterized by acute mental confusion, ataxia, and ophthalmoplegia. It is also a rare neurologic complication of hematopoietic stem cell transplantation (HSCT). However, because of its rare incidence, Wernicke's encephalopathy can easily be overlooked in HSCT patients, and a few misleading steps in the early stage of the disease may result in permanent neurologic disability or even mortality. We recently encountered a case of Wernicke's encephalopathy in a patient who underwent allogeneic HSCT. Based on our own experience and previously published documents, we suggest early radiologic surveillance and treatment for patients with findings compatible with Wernicke's encephalopathy following HSCT.
Go to : 
Wernicke's encephalopathy is named after Dr. Carl Wernicke, the Polish neurologist who first described the disease in 1881. Initially, the syndrome was characterized by a classic triad of acute mental confusion, ataxia, and ophthalmoplegia, but this triad was later found to occur in only 1/3 of the patients [1]. Wernicke's encephalopathy can be clinically diagnosed, when a patient shows central nervous system (CNS) symptoms compatible with radiologic evidence, especially on brain magnetic resonance imaging (MRI) [2]. This syndrome is well understood to be a consequence of thiamine deficiency and most commonly occurs in chronic alcoholics who are at risk of an unbalanced diet. However, it can occur under any condition that may induce malnutrition or malabsorption syndromes leading to thiamine deficiency [3, 4].
Hematopoietic stem cell transplantation (HSCT) does not seem to have a strong link with Wernicke's encephalopathy. However, HSCT can commonly cause anorexia, various degrees of stomatitis, graft-versus-host disease (GVHD), and infections involving the gastrointestinal tract, which lead to decreased oral intake and long-term use of total parenteral nutrition (TPN). Because commercialized TPN often lack thiamine, HSCT recipients are sometimes at risk of developing thiamine deficiency. However, despite the popular use of TPN agents during HSCT, only a few cases of HSCT-associated Wernicke's encephalopathy have been reported worldwide. We recently observed Wernicke's encephalopathy in a leukemia patient, who underwent allogeneic HSCT, and report the case here along with a review of previously reported cases.
Go to : 
A 45-year-old man diagnosed with secondary leukemia was admitted to our institute's hospital for allogeneic HSCT. He was first diagnosed with myelodysplastic syndrome (refractory cytopenia with multilineage dysplasia with a 9q deletion) 1.5 years ago. At 10 months after the initial diagnosis, the patient was readmitted because of dizziness, nausea, and abnormal complete blood counts revealing bicytopenia (hemoglobin, 7.2 g/dL; white blood cells, 1,200/µL; platelets, 342,000/µL). Bone marrow examination revealed that the erythroid elements were markedly increased up to 71.8% of all nucleated cells, with blasts up to 26.9% of non-erythroid cells. Based on the result, acute erythroid leukemia (AML-M6) was diagnosed. The patient was quickly treated with idarubicin and cytarabine for remission induction. Following successful induction treatment, the patient was prepared for allogeneic HSCT with his older sister, whose human leukocyte antigen fully matched his, as the donor.
Donor cluster of differentiation (CD) 34+ cells (2.68×106/kg) were collected after mobilization of peripheral blood stem cells with granulocyte-colony stimulating factor. After conditioning the patient with a classic busulfan and cyclophosphamide regimen, the prepared donor cells were infused into the patient without any acute complications. During the conditioning period, the patient developed grade 4 nausea and anorexia and could not receive oral nutrition. TPN was quickly applied for providing the much-needed nutritional support. The patient gradually recovered from neutropenia on day 12 of HSCT without any adverse events, and successful engraftment was confirmed by an engraftment assay performed on day 28 of HSCT. However, owing to continued loss of appetite and acute GVHD involving the gastrointestinal tract, TPN was maintained for over a month.
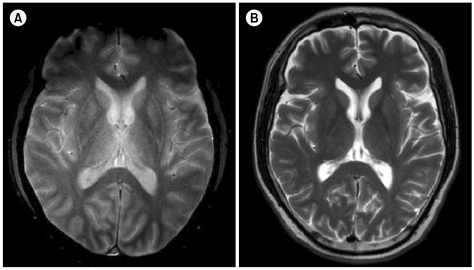
On day 48 of HSCT, the patient suddenly developed mental confusion, cognitive dysfunction, and asterixis. CNS examination with brain MR diffusion-weighted imaging revealed high signal intensities at the medial thalamus (Fig. 1A). Wernicke's encephalopathy was diagnosed based on the patient's history of consistent use of TPN, CNS symptoms, and typical radiologic findings, although the thiamine level was not checked. At the time of diagnosis, the cyclosporine level was 280.1 ng/mL, so calcineurin inhibitor-induced leukoencephalopathy was excluded. Thiamine was intravenously administered at a recommended dose of 1.5 g/day, resulting in rapid improvement of the CNS symptoms within 24 h of treatment initiation. The IV thiamine dose was maintained for 2 weeks and gradually reduced to peroral adminstered maintenance dose of 40 mg/day. Meanwhile, the patient recovered completely without any neurologic sequelae, and a follow-up brain MRI scan taken 2 weeks after the onset of Wernicke's encephalopathy showed reduced signal intensities in the thalamic areas (Fig. 1B). After a few more weeks of observation, the patient was discharged, and up to his most recent visit, he has shown no sign of recurrence.
Go to : 
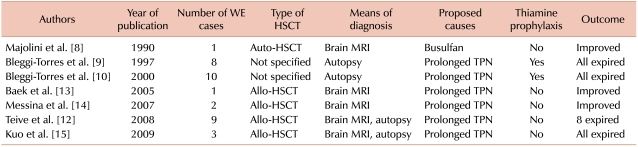
Thiamine (vitamin B1) plays a vital role in generation of energy needed for cerebral activities. It acts as a cofactor for enzymes like pyruvate dehydrogenase and α-ketoglutarate, which are essential in the Krebs cycle. Thus, thiamine deficiency leads to decreased activity of thiamine-dependent enzymes, which consequently leads to ineffective cerebral energy utilization and neuronal damage [5]. In these conditions, the classic symptoms of Wernicke's encephalopathy, such as ataxia, oculomotor dysfunction, confusion, and various degrees of cognitive dysfunction, may appear [4, 6]. Although almost all Wernicke's encephalopathy patients show some degree of improvement after initiation of thiamine replacement, only about 20% recover completely [4, 7]. Furthermore, mortality increases dramatically when treatment is delayed. It is not surprising that early reports show a high mortality rate, considering that the means for an early diagnosis were previously rare (Table 1).
Considering the frequency and amount of TPN needed during HSCT, it is not difficult to find the link between Wernicke's encephalopathy and HSCT. However, the occurrence of Wernicke's encephalopathy after HSCT is still a rare event, and only a few cases have been reported worldwide. Almost all published reports, except 1, concluded that prolonged TPN was the primary risk factor for HSCT-associated Wernicke's encephalopathy. The only other suggested cause was the use of busulfan in the conditioning regimen [8]. However, it is not yet clear whether other risk factors play a role in triggering such complications, the duration of TPN required for the disease to manifest itself is unknown.
Many authors have recommended the use of a thiamine supplement for the prophylaxis against Wernicke's encephalopathy. However, an earlier publication from a Brazilian group reported 8 patients who expired after developing Wernicke's encephalopathy despite using prophylactic thiamine (50 mg/day) [9]. Further studies are required to decide on an effective prophylactic dose of thiamine and to determine whether thiamine prophylaxis is effective in the prevention of Wernicke's encephalopathy in HSCT patients.
Initial CNS evaluation and management of the rarely occurring Wernicke's encephalopathy is not routinely recommended [7, 9-12]. However, we should remember that HSCT patients are always at risk of thiamine deficiency. Therefore, if an HSCT patient shows compatible signs and symptoms, then an early radiologic evaluation should be performed, and Wernicke's encephalopathy should be considered. Timely diagnosis and initiation of treatment are mandatory in order to minimize CNS complications and unwanted mortality.
Go to : 
References
1. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 1989. 2nd ed. Philadelphia, PA: F.A. Davis Co..
2. Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998; 171:1131–1137. PMID: 9763009.

3. Salmon DP. The Wernicke-korsakoff-syndrome and related neurologic disorders due to alcoholism and malnutrition (2nd ed.). J Stud Alcohol Drugs. 1991; 52:89–90.
4. Donnino MW, Vega J, Miller J, Walsh M. Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007; 50:715–721. PMID: 17681641.

5. Hazell AS. Astrocytes are a major target in thiamine deficiency and Wernicke's encephalopathy. Neurochem Int. 2009; 55:129–135. PMID: 19428817.

6. Buscaglia J, Faris J. Unsteady, unfocused, and unable to hear. Am J Med. 2005; 118:1215–1217. PMID: 16271902.

7. Azim W, Walker R. Wernicke's encephalopathy: a frequently missed problem. Hosp Med. 2003; 64:326–327. PMID: 12833822.
8. Majolino I, Caponetto A, Scimé R, Vasta S, Fabbiano F, Caronia F. Wernicke-like encephalopathy after autologous bone marrow transplantation. Haematologica. 1990; 75:282–284. PMID: 2227627.
9. Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, et al. Iatrogenic Wernicke's encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant. 1997; 20:391–395. PMID: 9339755.

10. Bleggi-Torres LF, de Medeiros BC, Werner B, et al. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000; 25:301–307. PMID: 10673702.

11. Cao XY, Wu T, Lu Y, Wang JB, Yin YM, Lu DP. A study of the central nervous system complications after hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi. 2010; 49:42–44. PMID: 20356481.
12. Teive HA, Funke V, Bitencourt MA, et al. Neurological complications of hematopoietic stem cell transplantation (HSCT): a retrospective study in a HSCT center in Brazil. Arq Neuropsiquiatr. 2008; 66:685–690. PMID: 18949262.

13. Baek JH, Sohn SK, Kim DH, et al. Wernicke's encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005; 35:829–830. PMID: 15750604.

14. Messina G, Quartarone E, Console G, et al. Wernicke's encephalopathy after allogeneic stem cell transplantation. Tumori. 2007; 93:207–209. PMID: 17557572.
15. Kuo SH, Debnam JM, Fuller GN, de Groot J. Wernicke's encephalopathy: an underrecognized and reversible cause of confusional state in cancer patients. Oncology. 2009; 76:10–18. PMID: 19018150.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download