Abstract
Invasive aspergillosis (IA) is a leading cause of infectious mortality in patients who have undergone a hematopoietic stem cell transplant (HSCT); the mortality due to IA ranges from 70% to 93% in HSCT patients. Early diagnosis and treatment are the cornerstones for the good prognosis of IA. Primary renal aspergillosis is an extremely rare presentation in patients who have undergone HSCT, and the risk factor for this uncommon presentation is not well known. We report a patient who developed primary renal aspergillosis and renal stones in both the kidneys after HSCT. Invasive renal aspergillosis was diagnosed after a nephrectomy, which was performed to treat massive renal hematoma.
Go to : 
Invasive aspergillosis (IA) is a major cause of morbidity and mortality in patients who have undergone a hematopoietic stem cell transplant (HSCT). The common sites of IA include lung, central nervous system, and paranasal sinuses. Primary renal aspergillosis is extremely rare; it has been mostly reported in immunocompromised patients. In Korea, only 3 cases of primary renal aspergillosis have been reported [1-3], but none were associated with HSCT. We report a patient who developed primary renal aspergillosis and renal stones in both the kidneys after HSCT. Invasive renal aspergillosis was diagnosed after nephrectomy, which was performed to treat massive renal hematoma.
Go to : 
A 33-year-old man was hospitalized after experiencing general weakness for 1 month. His medical history was unremarkable. Bone marrow examination revealed acute myeloid leukemia (AML). He received induction chemotherapy comprising continuous infusion of cytarabine (100 mg/m2) for 7 days and bolus injection of idarubicin (12 mg/m2) for 3 days. He achieved complete remission, and the same drugs were administered during consolidation chemotherapy. At the end of the first consolidation chemotherapy, he visited the outpatient clinic because of severe headache. Cerebrospinal fluid (CSF) cytology confirmed leukemia relapse in the central nervous system (CNS). Bone marrow examination did not show any evidence of hematologic relapse. Isolated CNS relapse was treated by intrathecal injection of methotrexate (12 mg; twice weekly). After the 4th injection, leukemic blasts in the CSF were unidentifiable. Therefore, methotrexate injection was discontinued after 2 additional courses. The patient received allogeneic peripheral blood SCT from a HLA-2-mismatched unrelated donor. Abdominal ultrasonography before the transplant had revealed renal stones with hydronephrosis in the right kidney. Since the patient had no specific symptoms related to renal stones, HSCT was continued without further treatment for renal stones. The conditioning regimen comprised busulphan (3.2 mg/kg; IV; 4 days) and cyclophosphamide (60 mg/kg; IV; 2 days). Tacrolimus and short-course methotrexate were administered for the prophylaxis of graft-versus-host disease (GVHD). Micafungin (50 mg daily; IV) was administered for anti-fungal prophylaxis from the 1st day of conditioning chemotherapy until engraftment. After engraftment, itraconazole (200 mg) solution was orally administered daily.
On the 19th day of transplantation, complete engraftment was achieved, and the absolute neutrophil count was 1,015/µL. The patient was discharged on day 27. Nonoliguric acute renal failure developed during the transplant period. The estimated glomerular filtration rate (as per Modification of diet in renal disease equation) was steady between 40 and 70 mL/min/m2; this was considered as a side effect of the calcineurin inhibitor. There were no symptoms and signs of acute GVHD. The serum was negative for Aspergillus galactomannan antigen, and itraconazole oral solution was substituted by fluconazole (100 mg; twice daily) peroral (PO) at the outpatient clinic.
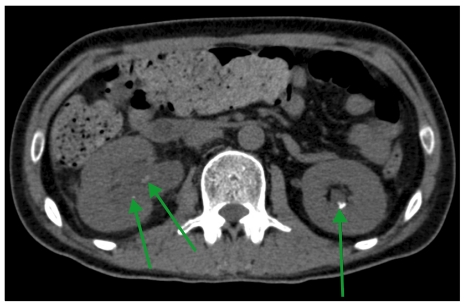
On day 74, he presented with right flank pain. Abdominal ultrasonography and non-contrast-enhanced abdominal computed tomography (CT) revealed multiple renal and ureteral stones with right hydronephrosis (Fig. 1) but no radiologic evidence of fungal infection in the kidney.
After urologic consultation, the patient was scheduled for extracorporeal shock wave lithotripsy (ESWL) for stone removal, but a febrile episode occurred. No pathogen was identified in the blood, sputum, and urine culture. PCR showed positive findings for cytomegalovirus (CMV) before the febrile episode, but the result of the consecutive test was negative without any anti-CMV treatment.
On day 75, serum analysis yielded positive findings for Aspergillus galactomannan antigen, and the consecutive test after 2 d also yielded positive findings (titer, 3.03). Non-contrast-enhanced chest CT and ostiomeatal unit CT showed no evidence of airway, pulmonary, or paranasal aspergillosis. Cefepime (2 g, IV) was administered twice daily for empirical broad-spectrum antibacterial therapy and amphotericin B deoxycholate (1 mg/kg/day) for empirical antifungal therapy. Subsequently, the fever subsided, and the patient underwent 2 courses of ESWL. The cumulative dose of amphotericin B deoxycholate reached 800 mg, and after discharge, the patient received itraconazole (400 mg) oral solution because the serum was still positive for Aspergillus galactomannan antigen.
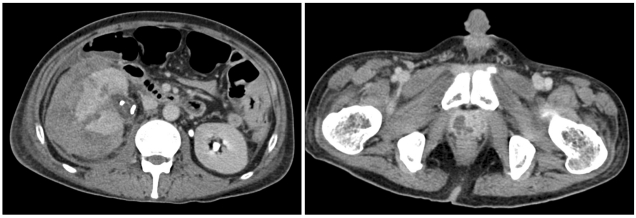
On day 96, the patient was admitted because of headache and right flank pain. Magnetic resonance imaging (MRI) of the brain revealed leptomeningeal leukemic infiltration, and CSF cytology confirmed the presence of CNS leukemia. Abdominal CT revealed residual multiple renal and ureteral stones in both kidneys. The serum Aspergillus galactomannan antigen titer was still high (3.32). For treatment of isolated CNS relapse, intrathecal methotrexate injection was administered twice. Ureteroscopic lithotripsy and ureteral stent placement were performed for stones in the right kidney and ureter. The right flank pain subsided immediately after ureteroscopy. However, 4 days later, the pain aggravated and fever developed. Abdominal CT showed a large subcapsular hematoma in the right kidney and abscess-like lesion in the prostate gland (Fig. 2). The WBC and platelet counts decreased to 150/µL and 33,000/µL, respectively.
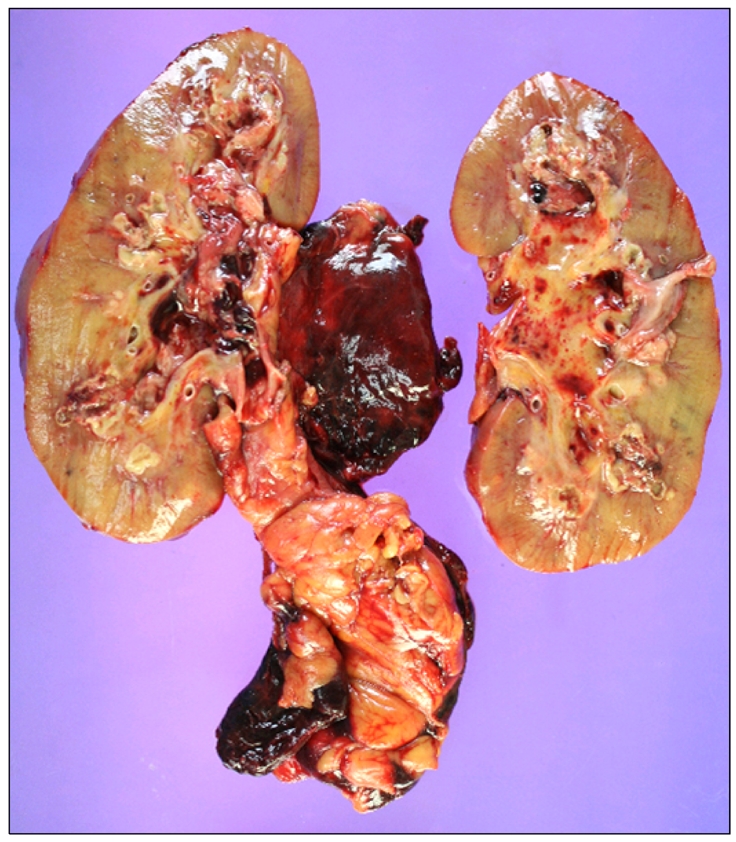
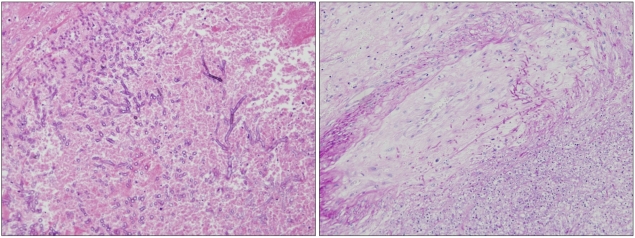
On day 109, emergency radical nephrectomy was performed (Fig. 3), and a ruptured area in the mid-portion of the lateral side of the right kidney was observed. Moreover, multiple fungal hyphae with septae and branching at acute angles in the renal parenchyma with vascular invasion, which was morphologically consistent with renal aspergillosis, were observed (Fig. 4). The identity of the causative fungal pathogen was inconclusive. The abscess-like lesion in the prostate was not manipulated because of the risk for hemorrhage. The patient was initially treated with meropenem and vancomycin for broad-spectrum antibacterial coverage and amphotericin B deoxycholate for empirical antifungal coverage after the development of fever. After confirmation of renal aspergillosis, voriconazole was administered instead of amphotericin B deoxycholate at the intensive care-unit after the operation, but the patient died on day 113 because of sepsis progression.
Go to : 
Aspergillus is a filamentous fungus occurring naturally worldwide. Aspergillus spp., Fusarium spp., and Pseudallescheria boydii show branched septate hyphae with angioinvasion, but Fusarium spp. show branching at right angles and constriction at their site of origin from parent hyphae and Pseudallescheria boydii show haphazard branching with prominent vesicles [4]. More than 900 species of Aspergillus exist, but less than 20 are pathogenic to humans [3]. Aspergillus fumigatus is the most common causative pathogen in invasive aspergillosis, followed by A. flavus, A. niger, and A. terreus [3].
IA is a leading cause of infectious mortality after HSCT. Although supportive care has improved and new antifungal agents and sensitive tests such as Aspergillus galactomannan antigen assay have been developed, mortality due to IA is high (70-93%) in HSCT patients [5]. The incidence of IA among the HSCT recipients has increased since the 1990s; this may be attributed to the grafts obtained from high-risk donors, increased profound immunosuppression, and prophylaxis with fluconazole, which is active against most Candida species but not against Aspergillus species. In recent years, IA has been the most common invasive fungal infection (IFI) following HSCT, accounting for 59% of IFIs [6].
In 2008, the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) classified IFI into proven, probable, or possible disease [7]. The Aspergillus galactomannan antigen test forms a part of the reference standard for diagnosing IA under the EORTC/MSG criteria. The sensitivity and specificity of this test were 71% and 89%, respectively, for proven cases, and 61% and 93% for proven and probable cases, respectively, in patients with neutropenia or other hematologic conditions [8], although false positive results may be obtained from patients receiving piperacillin, amoxicillin, or ticarcillin [9].
In our patient, the positive finding of serum galactomannan was first reported on day 75, and the subsequent tests were positive, with a continuously high titer. According to the EORTC/MSG classification, we diagnosed the patient with possible fungal infection and treated him with amphotericin B deoxycholate. Unfortunately, after 2 weeks of treatment, the serum galactomannan levels did not decrease, which may be attributed to the following reasons. First, although amphotericin B is used as a standard therapy for IA, only 13.3% of patients who had undergone allogenic HSCT had shown a favorable response [10]. Second, since amphotericin B is excreted mainly via the biliary tract (42%) and only a low percentage is excreted via the kidney (24%) [3], there is a possibility that the amphotericin B deoxycholate concentration is not sufficient to eliminate Aspergillus from the kidney. Finally, although Aspergillus species are rarely resistant to amphotericin B, the patient may have been infected by A. terreus, which is intrinsically resistant to amphotericin B. Itraconazole, which was administered subsequently after amphotericin B deoxycholate, is active against most Aspergillus species, including A. terreus. However, since itraconazole is mostly excreted in the feces, it may not be effective against renal aspergillosis. Therefore, surgical treatment of renal aspergillosis may achieve a good outcome [3]. If surgery cannot be performed, voriconazole and/or caspofungin can be administered, though the data on their efficacies are still limited [10, 11]. In our case, the patient had undergone radical nephrectomy, during which renal aspergillosis was incidentally observed. Extensive imaging studies were performed before surgery, but contrast non-enhanced CT scan and ultrasonography were not sufficient to identify the fungal lesions in the kidney. If IA is highly suspected in patients with kidney complications, aggressive diagnostic approaches, including contrast-enhanced imaging study (e.g., CT and MRI) may facilitate an accurate diagnosis and improve the treatment outcome.
In the only report of primary renal aspergillosis following HSCT, the patient had a stone in the renal pelvis [12]; this clinical presentation was similar to that observed in our patient. Many other reports have described the simultaneous presentation of renal aspergillosis and urinary tract stones. Haq et al. [13] reported primary renal aspergillosis in a diabetic patient with renal stones and Godec et al. and Eisenberg et al. reported cases with primary renal aspergillosis and obstruction at the ureteropelvic junction [14, 15]. These findings may suggest that urinary stasis induced by urinary tract obstruction may play some role in the development of renal aspergillosis.
In conclusion, the development of primary renal aspergillosis following HSCT is extremely rare, but if fungal infection is suspected in a patient with a urinary tract obstruction, especially with renal stones, renal aspergillosis must be considered in the differential diagnosis.
Go to : 
References
1. Kwon O, Woo JG, Yi JE, Jung GW, Cho S, Kim SR. A case of invasive aspergillosis limited to renal allograft. Korean J Nephrol. 2010; 29:300–304.
2. Na HH, Hong SW, Kim MC, Kang YK, Yoon YC, Koh HI. A case of invasive aspergillosis in transplanted kidney and perirenal area. J Korean Soc Transplant. 2008; 22:135–137.
3. Chung WB, Lee DG, Choi YK, et al. A case of isolated renal aspergillosis in a patient with acute myelocytic leukemia. Korean J Med. 2004; 67(Suppl 3):S845–S849.
4. Watts JC, Chandler FW. Connor DH, Chandler FW, Schwartz DA, editors. Aspergillosis. Pathology of infectious diseases. 1997. Vol. 2. Stamford, USA: Appleton & Lange;p. 933–941.
5. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007; 44:531–540. PMID: 17243056.

6. Chandrasekar PH, Ramesh M. Challenges in the management of invasive aspergillosis in hematopoietic stem cell transplantation. Expert Rev Anti Infect Ther. 2009; 7:1151–1153. PMID: 19968505.

7. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821. PMID: 18462102.

8. Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006; 42:1417–1427. PMID: 16619154.

9. Wheat LJ. Approach to the diagnosis of invasive aspergillosis and candidiasis. Clin Chest Med. 2009; 30:367–377. PMID: 19375641.

10. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347:408–415. PMID: 12167683.

11. Herbrecht R, Maertens J, Baila L, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant. 2010; 45:1227–1233. PMID: 20062093.

12. de Medeiros CR, Dantas da Cunha A Jr, Pasquini R, Arns da Cunha C. Primary renal aspergillosis: extremely uncommon presentation in patients treated with bone marrow transplantation. Bone Marrow Transplant. 1999; 24:113–114. PMID: 10435746.

13. Haq JA, Khan MA, Afroze N, Haq T. Localized primary renal aspergillosis in a diabetic patient following lithotripsy-a case report. BMC Infect Dis. 2007; 7:58. PMID: 17567923.

14. Godec CJ, Mielnick A, Hilfer J. Primary renal aspergillosis. Urology. 1989; 34:152–154. PMID: 2675456.

15. Eisenberg RL, Hedgcock MW, Shanser JD. Aspergillus mycetoma of the renal pelvis associated with ureteropelvic junction obstruction. J Urol. 1977; 118:466–467. PMID: 904060.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download