Abstract
Background
Combination treatment with all-trans-retinoic acid (ATRA) and anthracycline-based chemotherapy has led to major advances in the treatment of acute promyelocytic leukemia (APL).
Methods
In this study, we reviewed the outcome of pediatric APL patients treated using a modified AIDA protocol at our institution.
Results
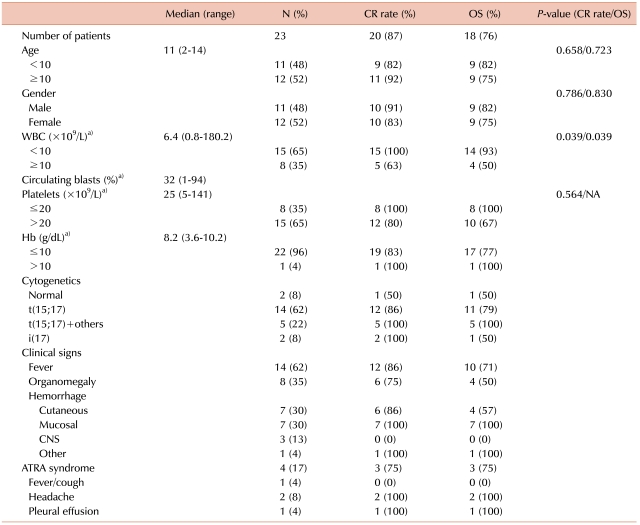
Between May 1999 and December 2007, 23 patients were diagnosed with APL at the Department of Pediatrics, Saint Mary's Hospital, The Catholic University of Korea. Eleven patients were male (48%) (median age at diagnosis, 11 (range, 2-14) years). The treatment protocol consisted of remission induction (achieved by coadministration of ATRA and idarubicin), 3 courses of consolidation treatment, and 2 years of maintenance treatment during which ATRA was also administered. Three patients died early during remission induction due to CNS hemorrhage. The remaining 20 patients achieved complete remission (CR), with an overall CR rate of 87%. Two patients relapsed and died, and another patient died of pneumonia unrelated to APL. Four patients (17%) were diagnosed with ATRA syndrome, and all patients showed resolution of symptoms. The event-free survival (EFS) and overall survival (OS) of the cohort were 78.3±8.6% and 76.3±9.5%, respectively. Initial WBC count at diagnosis was the only significant prognostic factor for the rate of CR (P=0.039) and OS (P=0.039).
Acute promyelocytic leukemia (APL) is different from other subtypes of acute myeloid leukemia (AML) because of the characteristic translocation between chromosomes 15 and 17 [1]. This translocation includes a breakpoint in the retinoic acid receptor, which leads to the creation of the promyelocytic leukemia/retinoic acid receptor α fusion protein (PML-RARα) [2]. Clinically, the disease is characterized by thrombocytopenia because of bone marrow insufficiency and coagulopathy at diagnosis [3].
As compared to other AML subtypes, APL responds well to anthracycline treatment. However, remission induction treatment consisting of anthracycline alone leads to early mortality from bleeding complications, which results in an overall lower complete remission (CR) rate than that for other AML subtypes.
However, since the first reports on the vitamin A derivative all-trans-retinoic acid (ATRA) in China in 1988, the CR rate of APL has reached 90%, and ATRA efficacy has been further proven in several clinical studies [4-6]. However, ATRA administered alone leads to a high rate of relapse within a year of CR, while ATRA combined with intensive chemotherapy results in improved CR rate and overall survival (OS) [6-8]. In 1996, Avvisati et al. [9] first introduced the AIDA (ATRA plus idarubicin) protocol, and since then, maintenance chemotherapy using ATRA has led to improved OS.
In our institution we implemented a modified AIDA protocol for children newly diagnosed with APL; our protocol consisted of combined ATRA and anthracycline-based chemotherapy for remission induction and consolidation, followed by regular administration of ATRA during maintenance therapy. In this study, we analyzed the outcome of patients diagnosed with APL and treated according to the modified AIDA protocol at our institution.
Twenty-three patients diagnosed with APL from May, 1999 to December, 2007 at the Department of Pediatrics, Saint Mary's Hospital, the Catholic University of Korea, were included in the study. All patients were newly diagnosed, with morphology of bone marrow aspirate consistent with APL. Fluorescent in situ hybridization (FISH), RT-PCR and immunophenotype studies were performed in all patients, and t(15;17)(q22;q12) was identified. Patients with isochromosome 17 were also included in the study.
Patients newly diagnosed with APL were given ATRA at a dose of 45 mg/m2/day divided in 2 doses. To prevent the occurrence of ATRA syndrome, oral dexamethasone was administered at a dose of 8 mg/m2/day divided in 3 doses for 3 days from the start of ATRA. Idarubicin administration at a dose of 12 mg/m2/day was started on day 2, and was also administered on days 4, 6, and 8 for a total of 4 doses.
After remission induction, consolidation chemotherapy was initiated in a 3-step manner. During initial consolidation, cytarabine and idarubicin were administered for 4 consecutive days at doses of 1 g/m2/day and 5 mg/m2/day respectively. In the second step of consolidation, mitoxantrone 10 mg/m2/day was administered on days 1-5, and etoposide 100 mg/m2/day was administered on days 1-4. During the third and final steps of consolidation, idarubicin was administered at a dose of 12 mg/m2/day on day 1, and cytarabine at a dose of 100 mg/m2/day on days 1-5.
After each course of consolidation, bone marrow examination was performed to verify CR status. Maintenance chemotherapy was started after completion of consolidation therapy and included ATRA (45 mg/m2/day) for 14 days every 3-month period, oral methotrexate (20 mg/m2/day) once weekly, and 6-mercaptopurine given daily (60 mg/m2/day) for 2 years. Scheduled bone marrow examinations were also performed during maintenance therapy to assess CR status.
A retrospective analysis of the clinical course of patients was performed. Basic demographics were recorded, along with dates of first complete remission, relapse and death. Cytogenetic abnormalities as diagnosed on metaphase bone marrow chromosomal studies were recorded. In addition the presence of ATRA syndrome during treatment was noted. ATRA syndrome was defined as the presence of at least 3 of the following signs concurrent with the administration of ATRA in the absence of other causes: fever, weight gain, respiratory distress, lung infiltrates, pleural or pericardial effusion, hypotension, and renal failure [8]. The analyses were performed as of April 30th, 2010.
Chi-square test was used to compare categorical variables. Event-free survival (EFS) and OS were calculated using the Kaplan-Meier method, and analysis of risk factors for CR rate and OS was done using the Cox proportional hazards model. For multivariate analysis, factors known to affect clinical outcome, such as age, gender, initial WBC count and platelet count, were entered into the model. P-values <0.05 were regarded as statistically significant.
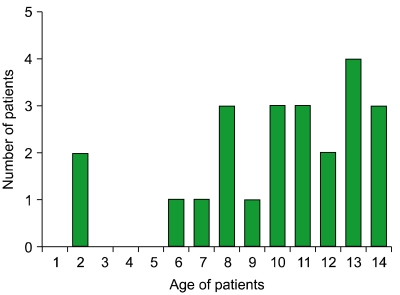
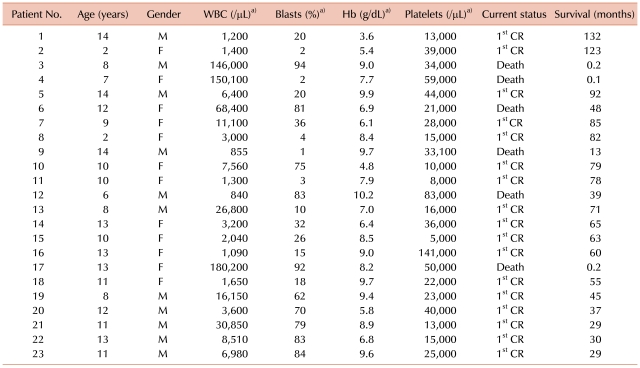
The major characteristics of the 23 patients are outlined in Table 1. Apart from 1 patient who had been previously diagnosed with Fanconi anemia, none of the others had a known history of hematologic disease at diagnosis. The Fanconi anemia patient has completed the treatment for APL, and is currently alive with no major events. Eleven patients were males (48%) and 12 were females (52%). Median age at diagnosis was 11 years (range, 2-14). The age distribution of the patients is shown in Fig. 1.
The most common signs or symptoms at diagnosis were bleeding tendency (N=18, 78%), fever (N=14, 61%), and hepatosplenomegaly (N=8, 35%). The median WBC count at diagnosis was 6,400/µL (range, 840-180,200), with 15 patients (65%) presenting with an initial WBC count <10,000/µL, and 8 patients (35%) ≥10,000/µL (Table 2).
Chromosomal studies showed a normal karyotype in 2 patients (8%) (Table 2), who were diagnosed by bone marrow aspiration morphology and by identification of PML/RARα fusion gene with FISH studies. Isochromosome 17, which has been associated with a poor prognosis [10, 11], was identified in 2 patients, one of whom relapsed and died.
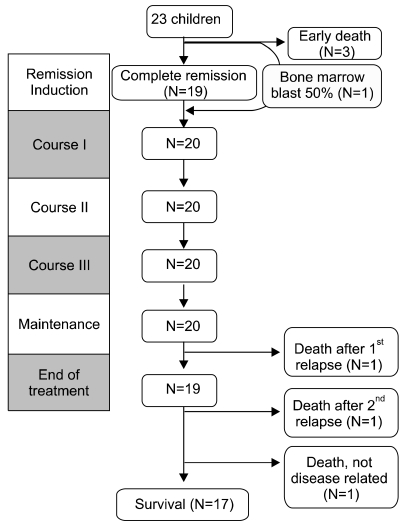
Three patients died after initiation of remission induction chemotherapy (13%), the cause of early death being CNS hemorrhage in all cases. One patient died after a single infusion of idarubicin, while the remaining 2 patients died before the initiation of anthracycline chemotherapy. The average duration of survival after diagnosis was 4.3 days (range, 3-7) in these 3 patients.
After initial remission induction treatment, 19 of the surviving 20 patients achieved CR (83%). The remaining patient was found to have 50% myeloblasts on follow-up bone marrow study, but progressed to the first cycle of consolidation, after which CR was diagnosed. Hence, overall CR rate was 87% with a median duration of 33 days till diagnosis of CR (range, 27-79) (Table 2).
A total of 20 patients completed the consolidation phase of treatment and progressed to the maintenance phase. Three of these 20 patients died; 1 patient relapsed after 12 months of maintenance treatment and died during reinduction treatment. One patient relapsed 28 months after the end of treatment, achieved a second CR, but showed CNS relapse 6 months later. The patient underwent autologous stem cell transplantation without achieving a third CR, and died of sepsis afterwards. Another patient died of pneumonia 70 days after completing treatment for APL. The remaining 17 patients are alive without relapse, and their course is being followed (Fig. 2).
ATRA syndrome was diagnosed in 4 patients (17%) (Table 2), 3 patients during remission induction and the remaining 1 patient during maintenance treatment. One patient who presented with fever and cough showed improved symptoms after the tapering of ATRA dosage, while another patient who showed pleural effusion was given a second course of dexamethasone. The 2 patients who complained of headache, one of whom experienced ATRA syndrome during maintenance chemotherapy, were both treated with dexamethasone.
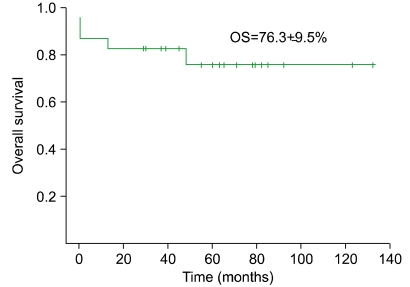
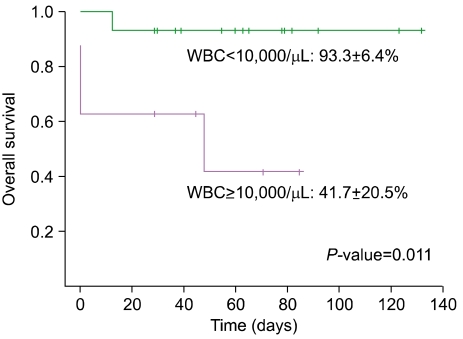
The EFS of the cohort was 78.3±8.6%. Similarly, the OS was 76.3±9.5% (Fig. 3), with a median duration of survival of 61.5 months (range, 13-132) excluding the 3 patients who experienced early death. The initial WBC count proved to be significant for the prognosis of CR (P=0.039), while other variables such as age, gender, and initial platelet count did not have a significant impact on univariate study. The multivariate analysis confirmed that the initial WBC count was the only factor with statistical significance on CR (HR 5.49, 95% CI 1.40-21.55, P=0.015). Similarly, WBC count was the only factor with statistical significance on OS (P=0.039) as shown by univariate analysis and Kaplan-Meier survival estimate (Fig. 4).
The main purpose of our study was to investigate the rates of CR and OS of children diagnosed with APL, who were treated with a combined ATRA and chemotherapy regimen incorporated into initial remission induction, consolidation and maintenance therapy.
In children, EFS and OS rates of AML are reported to be 45±4% and 56±4% respectively, which are lower than those reported for ALL [12]. APL comprises 8-12% of pediatric AML, and, as in the adult population, is characterized by disseminated intravascular coagulation resulting in bleeding and death [13, 14].
Since the introduction of ATRA, a combined chemotherapeutic approach using ATRA and other anti-neoplastic agents has been implemented, resulting in CR rates of 86-92% and EFS rates of 62-82%, as has been shown by the European APL study, the GIMEMA and PETHEMA studies [8, 15, 16]. Furthermore, clinical research has shown positive effects of ATRA in resolving the bleeding tendency inherent in newly diagnosed APL. Since 1999, we have treated APL patients with ATRA and idarubicin, according to the AIDA protocol, which has resulted in high EFS and OS rates for children with APL [15]. The results of our study, which included patients treated during a 9 year period also corroborate the efficacy of this regimen with high CR (87%) and OS (76%) rates.
Previous studies on risk factors in APL have highlighted the importance of initial WBC and platelet counts, as well as patient gender on overall prognosis [17, 18]. However, in our study initial WBC count was found as the only variable with prognostic significance in multivariate analysis.
Early after initiation of treatment, 3 patients died of CNS hemorrhage comprising 60% of disease-related mortality. Aggressive prevention and treatment of bleeding at the beginning of chemotherapy remains a key factor in overall treatment success.
APL is characterized by a translocation between chromosomes 15 and 17, which results in the fusion of PML gene on the long arm of chromosome 15 with RARα gene on the long arm of chromosome 17. A variant of this translocation is isochromosome 17, and patients with this variant were also included in this study. Isochromosome 17 is rarely reported in the literature, but is known to have a poor prognosis in the adult population [19, 20]. However, the limited number of patients with this chromosomal variant in our study precluded the possibility of making definite conclusions on this subject.
Complications resulting from ATRA treatment are commonly referred to as "ATRA syndrome," an entity that includes leukocytosis, fever, weight gain, respiratory failure, pleural or pericardial effusion, and renal failure [21, 22]. The reported incidence of ATRA syndrome varies from 2% to 30%, with a mortality of 1-8% [23, 24]. Treatment options for this complication include a reduction in the dose of ATRA or coadministration of dexamethasone [24, 25]; in our modified AIDA protocol, dexamethasone was concomitantly administered for 3 days after the start of ATRA as a prophylaxis of ATRA syndrome. As a result, ATRA syndrome was diagnosed in 4 patients, including 3 patients in remission induction, which indicated the potential efficacy of dexamethasone for the prevention of ATRA syndrome. Signs and symptoms of the ATRA syndrome were mild headache, pleural effusion, fever and cough, but all patients showed resolution of the symptoms after either restarting dexamethasone or decreasing ATRA dosage.
Our results, derived from a small cohort of patients, indicate that combination of ATRA and chemotherapy during both remission induction and maintenance therapy led to high rates of CR and OS, consistent with the results that have previously been reported. Active prevention of CNS bleeding during the initiation of treatment may limit early mortality, while identification of patients with high initial WBC counts as a high-risk group may lead to superior survival outcomes. The limited number of pediatric patients in this study, when compared to those done on the adult population, emphasize the need for a larger scale study in order to draw conclusions about the impact of significant risk factors, such as ATRA syndrome, on clinical endpoints.
References
1. Avvisati G, Lo Coco F, Diverio D, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell' Adulto (GIMEMA) pilot study. Blood. 1996; 88:1390–1398. PMID: 8695858.
2. Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999; 93:3167–3215. PMID: 10233871.
3. Tallman MS, Hakimian D, Kwaan HC, Rickles FR. New insights into the pathogenesis of coagulation dysfunction in acute promyelocytic leukemia. Leuk Lymphoma. 1993; 11:27–36. PMID: 8220153.

4. Kim SG, Han CW, Kim YJ, et al. Effect of all-trans retinoic acid (ATRA) on the remission induction and coagulopathy in acute promelocytic leukemia (APL). Korean J Med. 1997; 53:199–206.
5. Bapna A, Nair R, Tapan KS, et al. All-trans-retinoic acid (ATRA): pediatric acute promyelocytic leukemia. Pediatr Hematol Oncol. 1998; 15:243–248. PMID: 9615322.

6. Cho YY, Kim JG, Chae YS, Moon JH, Ahn BM, Sohn SK. Salvage treatments with all-trans retinoic acid and arsenic trioxide-based regimens in acute promyelocytic leukemia. Korean J Med. 2008; 74:596–604.
7. Sanz MA, Martín G, Rayón C, et al. PETHEMA group. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. Blood. 1999; 94:3015–3021. PMID: 10556184.
8. de Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004; 22:1404–1412. PMID: 15084614.

9. Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997; 337:1021–1028. PMID: 9321529.

10. Kim M, Lee SA, Park HI, et al. Two distinct clonal populations in acute promyelocytic leukemia, one involving chromosome 17 and the other involving an isochromosome 17. Cancer Genet Cytogenet. 2010; 197:185–188. PMID: 20193853.

11. Park JP, Fairweather RB, Mohandas TK. Isochromosome for derivative 17q in acute promyelocytic leukemia: evidence for two copies of PML-RARA and favorable response to all-trans-retinoic acid therapy. Genes Chromosomes Cancer. 1997; 18:151–153. PMID: 9115966.

12. Rubnitz JE. Childhood acute myeloid leukemia. Curr Treat Options Oncol. 2008; 9:95–105. PMID: 18506629.

13. Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007; 92:1519–1532. PMID: 18024401.

14. Stone RM, Mayer RJ. The unique aspects of acute promyelocytic leukemia. J Clin Oncol. 1990; 8:1913–1921. PMID: 2230879.

15. Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOP AIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005; 106:447–453. PMID: 15677559.
16. Ortega JJ, Madero L, Martín G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005; 23:7632–7640. PMID: 16234524.
17. Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002; 99:759–767. PMID: 11806975.

18. da Costa Moraes CA, Trompieri NM, Cavalcante Felix FH. Pediatric acute promyelocytic leukemia: all-transretinoic acid therapy in a Brazilian pediatric hospital. J Pediatr Hematol Oncol. 2008; 30:387–390. PMID: 18458575.

19. Manola KN, Karakosta M, Sambani C, et al. Isochromosome der(17)(q10)t(15;17) in acute promyelocytic leukemia resulting in an additional copy of the RARA-PML fusion gene: report of 4 cases and review of the literature. Acta Haematol. 2010; 123:162–170. PMID: 20224268.

20. Im SA, Kim SH, Lee MA, et al. Identification of ider[17q] in addition to t[15;17] in acute promyelocytic leukemia using whole chromosome painting probes made by interspecies hybrid using inter-Alu PCR. Cancer Genet Cytogenet. 2000; 118:169–170. PMID: 10798868.
21. Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr. The "retinoic acid syndrome" in acute promyelocytic leukemia. Ann Intern Med. 1992; 117:292–296. PMID: 1637024.

22. de Botton S, Chevret S, Coiteux V, et al. Early onset of chemotherapy can reduce the incidence of ATRA syndrome in newly diagnosed acute promyelocytic leukemia (APL) with low white blood cell counts: results from APL 93 trial. Leukemia. 2003; 17:339–342. PMID: 12592333.

23. Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008; 33:331–338. PMID: 18613850.

24. Larson RS, Tallman MS. Retinoic acid syndrome: manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol. 2003; 16:453–461. PMID: 12935962.

25. Asami K, Sasazaki Y, Utsumi J. Successful treatment of retinoic acid syndrome with high-dose dexamethasone pulse therapy in a child with acute promyelocytic leukemia treated with ATRA. Acta Paediatr Jpn. 1995; 37:384–387. PMID: 7645395.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download