IDENTIFICATION OF MESENCHYMAL STEM CELLS AS REPLACEMENT CELLS
Historical studies have shown that bone marrow contains non-hematopoietic cells in addition to hematopoietic cells. The non-hematopoietic cells adhere to plastic dishes and can differentiate into mesenchymal tissues such as osteoblasts, chondrocytes, adipocytes, or myoblasts, and are hence referred to as mesenchymal stem cells. However, the unfractionated mesenchymal cells obtained from bone marrow or other tissues are characterized by extensive heterogeneity, and cell populations that can satisfy the criteria for being stem cells are very rare. Therefore, to clarify the gap between nomenclature and function, the International Society for Cell Therapy 2005 has adopted the term "mesenchymal stromal cells (MSCs)" rather than mesenchymal stem cells.
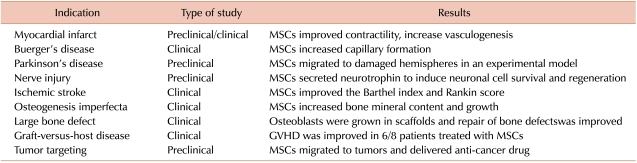
Notably, these plastic-adherent MSCs can be easily cultured in vitro and expanded to a clinical scale. In addition, the multilineage differentiation observed in MSCs has enabled therapeutic trials to repair various kinds of tissue damage using ex vivo-expanded MSCs. These trials included cell therapy for regeneration of myocardium in ischemic myocardial infarct, regeneration of blood vessels in Buerger's disease, repair of nerve tissues in traumatic injury or ischemic cerebral stroke, regeneration of bones in osteogenesis imperfecta or in large bone defects, as well as some immunological applications, such as amelioration of graft-versus-host disease (Table 1) [1]. While these trials using MSCs provided some encouraging results, the extent of functional improvement or the contributions of MSCs to the structure of the regenerated tissues have not been as satisfactory as initially anticipated. For example, while large numbers of experimental studies have demonstrated the differentiation of MSCs into "myocardium-like cells" expressing myocardium-specific markers, few studies have demonstrated their successful differentiation into mature myocardium, or their functional integration into the damaged myocardium. Similarly, MSCs have been shown to differentiate into various types of neuronal tissues but very rarely acquire synaptic connections into pre-existing neuronal tissues. Moreover, the structural contributions of MSCs as building blocks in the regenerated tissues have been limited because of their limited survival and the half-life of the transplanted cells. Thus, in most trials using MSCs, the major therapeutic benefit does not appear to result from their direct replacement of damaged cells.
NEW UNDERSTANDING OF MSCS AS CELLS FOR REGENERATIVE MICROENVIRONMENT
While contributions of MSCs by direct differentiation into specific types of tissue were limited, MSCs were shown to secrete a variety of molecules, including bioactive and extracellular matrix factors. Interestingly, the secretion of bioactive factors by MSCs was regulated in a manner tightly associated with their growth and differentiation, i.e., each cellular condition of MSCs gave rise to a distinct set of secreted factors. These observations suggested that one of the primary and key functions of MSCs is to secrete large amounts of bioactive molecules in response to local environmental conditions [2]. Moreover, MSCs have been shown to migrate to the site of local tissue injury or inflammation, penetrating across the endothelial layers of vessels; this migration is dependent on the specific interaction of adhesion molecules such as P-selectin or VCAM-1 in endothelial cells. Taken together, these observations now suggest that the therapeutic effects of MSCs may be specific delivery of bioactive factors that can facilitate the regeneration of tissues at the site of local injury. These effects, termed "trophic activity" by A. Caplan [2], exhibit a common mode of action of bioactive molecules, i.e., (1) inhibition of apoptosis and limitation of the field of damage or injury; (2) inhibition of fibrosis or scarring at sites of injury; (3) stimulation of angiogenesis to bring in new blood supply; and (4) stimulation of mitosis of tissue-specific and "endogenous" stem cells. Other secretary factors include factors for immune modulation to inhibit activation of T-cells, chronic inflammatory processes, or autoimmune reactions. Furthermore, MSCs secrete factors that can promote vasculogenesis, such as VEGF. Thus, it is possible that the intrinsic function of MSCs is not to replace damaged cells but rather to provide a regenerative microenvironment.
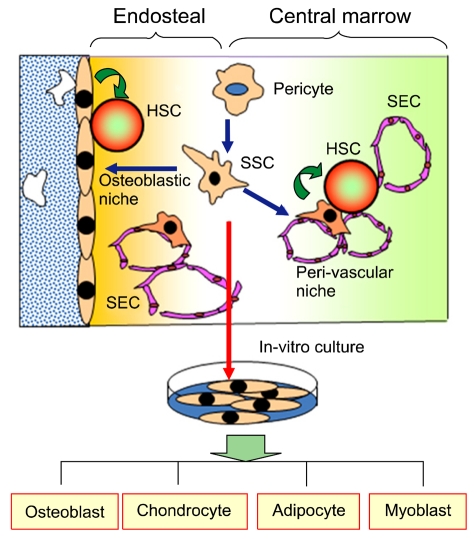
In fact, the hypothesis that MSCs may comprise a regenerative microenvironment for tissue-specific stem cells has been supported by studies on the bone marrow model. It was shown that hematopoietic stem cells (HSCs) reside in a specialized structure of bone marrow, called niche, either in the endosteal or perivasular region. The endosteal niche is comprised mainly of osteoblasts in the endosteal surface of trabecules, whereas the perivascular niche is comprised mainly of reticular cells adjacent to sinus endothelial cells (SECs). Notably, recent studies have shown that a specific population of MSCs that express CD146 can form colony-forming units of fibroblasts (CFU-Fs) and retain the potential to regenerate both endosteal and perivascular niches. These findings strongly suggest that the cellular structures for both types of niches originate from a common source, which was referred to as skeletal stem cells (SSCs) (Fig. 1) as reviewed by in our previous study [3]. On the other hand, a recent study demonstrated that pericytes located in the peri-vascular areas of various organs were analogous to MSCs, capable of differentiating into multiple cell types such as osteoblasts, adipocytes, and myoblasts. Thus, one striking inference about the in vivo identity of MSCs is that they may be derived from pericytes, as hypothetically illustrated in Fig. 1. Importantly, these pericytes are released from vascular structures in the case of focal injury, permitting them to function as repair cells on the site. Altogether, it appears that MSCs are an in vivo counterpart of pericytes that constitute the stromal structure of the stem cell niche for HSCs [3]. Thus, an integrated view of the emerging understanding on the role of MSCs might be that MSCs function as a local stem cell niche to facilitate the regeneration of tissue-specific stem cells, rather than functioning as replacement cells in the site.
FUTURE DIRECTIONS AND SAFETY CONSIDERATION
This new insight on MSCs, that they function as a regenerative microenvironment, opens an exciting field of future studies, specifically the dissection of the cross-talk that may occur between the mesenchymal niche cells and tissue-specific stem cells at the injury site. These interactions between the MSC niche and tissue-specific stem cells and their signals are now being actively revealed, as reviewed in our recent study [4]. Therefore, dissecting the cross-talk in the stem cell niche may help identify strategies to coax an optimal microenvironment in the tissue, which will then boost the use of MSCs in cell therapy. On the other hand, it should be also mentioned that safety is the most important factor to be considered. While MSCs have been found to be safe in most clinical trials, it was shown that the size of ex vivo cultured MSCs vary from 15-55 µm in diameter, apparently larger than the pulmonary capillary. Therefore, several studies using animal models showed that systemic intra-vasculature injection of MSCs could induce pulmonary embolisms in the recipients [5]. In addition, inhibitory effects of MSCs on immune functions have raised caution that MSCs can provoke tumor growth in recipients. Thus, additional caution with regard to the safety of MSCs, as well as new approaches in their therapeutic use, is necessary to ensure therapeutic benefits of MSCs and to overcome its intrinsic risk in cell therapeutic trials. Finally, experts worldwide are warning against the current trend of medical tourism to underdeveloped countries for stem cell injections, without sufficient safeguards against possible accidents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download