INTRODUCTION
Acute promyelocytic leukemia (APL) is a specific type of acute myeloid leukemia (AML) characterized by the presence of abnormal promyelocytes in bone marrow and peripheral blood, young age at presentation, specific chromosomal abnormalities, coagulopathy, and a unique response to treatment with retinoic acid. Until recently, combination of all-trans retinoic acid (ATRA) and chemotherapy with cytarabine and anthracyclines has been the standard treatment for APL. Although treatment with ATRA has improved the outcome of APL, early bleeding due to coagulopathy and relapse are the main causes of death in patients with APL [1].
APL may relapse in central nervous system (CNS). However, CNS involvement at relapse is rare and is generally associated with a poor prognosis. We present a case of long-term survival in an APL patient who had achieved hematologic and molecular complete remission (CR) after treatment of meningeal relapse using ATRA, idarubicin and cytarabine.
CASE REPORT
A 39-year-old woman presented with headache and petechiae. Her complete blood count (CBC) showed a white blood cell (WBC) count of 8,600/µL consisting of 75% blasts, hemoglobin level of 9.4 g/dL, and platelet count of 13,000/µL. Coagulation test showed prothrombin time of 13.5 sec, partial thromboplastin time of 26.1 sec, and fibrinogen level of 80 mg/dL. APL was diagnosed on the basis of findings from bone marrow (BM) examination, and was confirmed by chromosomal analysis, which indicated the presence of t(15;17) (q22;q11.2) and molecular analysis, which indicated the presence of promyelocytic leukemia (PML)/retinoic acid receptor-a (RARA) rearrangement. Induction chemotherapy with idarubicin (intravenous administration of idarubicin 12 mg/m2/day on days 2, 4, 6, and 8) and ATRA (45 mg/m2) was administered to achieve CR. BM examination revealed normal karyotype and absence of the PML/RARA rearrangement. The patient received consolidation therapy with 2 consecutive courses of idarubicin and 1 course of mitoxantrone, followed by maintenance therapy with ATRA (45 mg/m2 on days 1-15, every other month for 2 years).
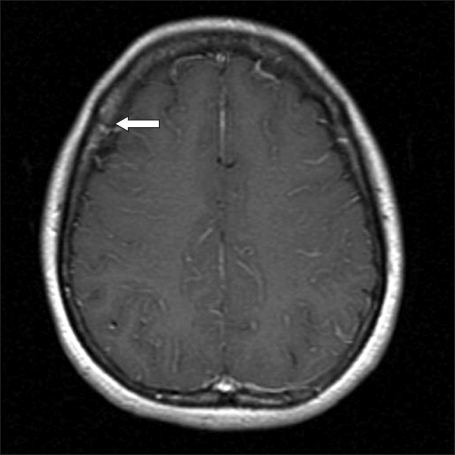
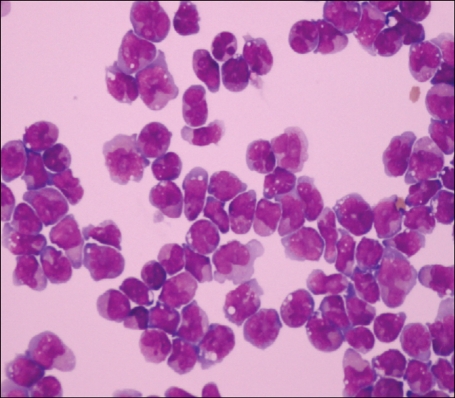
After 17 months, the patient complained of non-specific headache for 1 month. Her physical examination was unremarkable and her CBC was normal. Enhanced T1-weighted magnetic resonance image showed focal meningeal enhancement in the right parietal lobe (Fig. 1). Cerebrospinal fluid (CSF) microscopic finding showed numerous leukemic promyelocytes-containing prominent nuclear irregularities and variably granulated cytoplasm (Fig. 2) and PML/RARA rearrangement was detected. BM study showed hematologic, cytogenetic, and molecular CR. The patient was diagnosed with meningeal relapse of APL and treated with intrathecal administration of methotrexate and cytarabine (methotrexate 12 mg and cytarabine 50 mg administered 3 times a week until the WBC count in the CSF reached zero), followed by whole-brain radiotherapy and systemic chemotherapy (cytarabine 2.0 g/m2 I.V. b.i.d. on days 1-3, etoposide 70 mg/m2 I.V. on days 1-4). The patient has survived for 68 months since the last systemic chemotherapy.
DISCUSSION
Extramedullary involvement at relapse is rarely observed in patients with APL. Skin, CNS, middle ear, lung, lymph node, mediastinum, thymus, spine, breast, pelvis, and gingival are the common extramedullary sites. The skin is the most common extramedullary site [2].
The symptoms of CNS involvement in APL are headache, vertigo, nausea, vomiting, visual disturbance, motor weakness, and seizure. In our case, the patient complained of non-specific headache. Although meningeal infiltration of leukemia is rare, CSF should be examined when symptoms of suspected CNS involvement are present. Interestingly, in our patient, CNS relapse did not coincide with hematologic and molecular relapse. Among the reported cases of APL, 6 continued to show hematologic and molecular remission despite CNS relapse [3-7].
ATRA induces differentiation of abnormal promyelocytes into mature granulocytes and induces CR in 80-90% of newly diagnosed and first-relapsed cases of APL [1, 8, 9]. After the advent of ATRA treatment, an increasing number of APL cases with the involvement of extramedullary sites at relapse have been reported. Thus, ATRA treatment may predispose APL patients to extramedullary relapse. Several biologic effects such as ATRA-mediated increase in the expression of certain adhesion molecules (e.g., CD11c, CD13, and CD56) have been suggested to play a role in extramedullary involvement of leukemic cells [10, 11]. A GIMEMA study compared the relapse pattern in APL patients receiving different treatments (ATRA+chemotherapy group vs. chemotherapy alone). Unlike patients receiving chemotherapy alone, patients receiving ATRA and chemotherapy did not have an increased risk of involvement of extramedullary sites at relapse. However, patients receiving ATRA had a higher prevalence of CNS involvement. These findings suggest that with the advent of ATRA and subsequent combination chemotherapy, a substantially higher fraction of patients who achieve long-term remission are at the risk of developing CNS relapse than patients who were treated with chemotherapy alone in the past [12].
The prognosis of CNS relapse in APL is generally poor. In particular, patients with multifocal CNS involvement had short survival time [3, 8]. Our findings indicate that after multimodal treatment, patients with focal involvement of the CNS have longer survival times than patients with multifocal involvement of the CNS. Treatment of CNS relapse consists of intrathecal chemotherapy, radiation therapy, systemic chemotherapy with high-dose cytarabine, and treatment with agents such as ATRA. Although ATRA does not cross the blood-brain-barrier (BBB), it induces the differentiation of APL blasts present in the CSF of patients with CNS relapse [13]. High-dose cytarabine and arsenic trioxide (AS2O3) pass through the BBB and could be effective for APL patients with CNS relapse [14, 15].
In summary, we presente a case of long-term survival in an APL patient with isolated meningeal relapse. CNS is the possible site of relapse in patients with CR after initial treatment. Therefore, precise evaluation and diagnosis by using approaches such as lumbar puncture and CSF examination should be performed, if CNS symptoms occur in patients with APL.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download