INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) has been widely performed for various genetic and acquired as well as malignant and non-malignant diseases. However, highly intensified conditioning regimens can lead to serious morbidity and mortality such as major organ dysfunction, life-threatening infections, and bleeding. In allogeneic HSCT, graft-versus-host disease (GVHD) constitutes a major additional source of transplant-related morbidity and mortality. Neurological complications are also significant clinical problems in the posttransplant period in allogeneic HSCT recipients.
Significant neurological events have been variably reported to range from 11% to 59% of HSCT recipients and to be associated with a mortality rate up to 10% of patients [
1-
4]. Cyclosporine (CsA)-related neurotoxicity or metabolic disturbances have been reported to be one of the most common neurological complications [
2,
3,
5-
7], whereas in autopsy studies, cerebrovascular disorders were the primary diagnosis in the neuropathological examination [
8]. Despite the fact that neurological complications after allogeneic HSCT represent a major cause of morbidity and mortality, they have not been clearly described, especially in the pediatric population. Furthermore, the risk factors for neurological complications and the impact of neurological complications on posttransplant survival have been inconsistently described.
We evaluated the incidence of significant neurological complications occurring in the early period after HSCT by focusing on the central nervous system (CNS) complications. The main objectives were to describe the various types of CNS complications, the impact of different risk factors, and the survival outcomes according to CNS complications in allogeneic HSCT for children.
MATERIALS AND METHODS
1. Patients
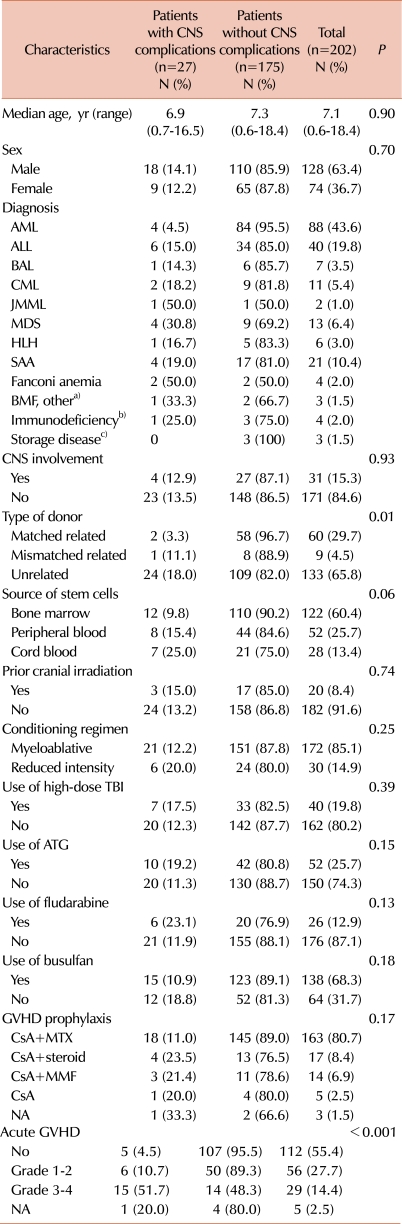
This study included 202 consecutive patients who underwent allogeneic HSCT for malignant and non-malignant hematologic disorders and inherited disorders at Asan Medical Center (Seoul, Korea) between June 1997 and August 2009. All patients were younger than 20 years at the time of transplantation. Data on demographics, underlying diseases, CNS involvement of the primary disease, CNS irradiation before treatment, transplant-related factors, and clinical course after transplantation were collected retrospectively from the transplant database and electronic medical records of Asan Medical Center.
Histocompatibility of all donor-recipient pairs was determined by serology for human leukocyte antigens (HLA)-A, -B, and -DRB1. Patients and donors were typed for HLA-A, -B, and -DRB1 by serological methods between 2000 and 2002 and by high-resolution molecular typing since 2003. A fully matched sibling was defined as a 6/6 match on all A, B, and DRB1 loci. Unrelated donors were 6/6-matched, 8/8 allele-matched, or mismatched at a single antigenic or allelic locus.
2. CNS complications
Data were retrieved on clinically significant CNS complications occurring within the first 6 months following allogeneic HSCT. For the purpose of this study, only toxicities of grade 3 or 4 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 were considered. The following complications were defined as clinically significant: seizures, altered level of consciousness, visual disturbances, involuntary movements, motor/sensory deficits, cranial nerve palsies, and severe headaches. CNS relapse and CNS events that occurred following relapsed disease were not included. Non-repetitive seizures associated with busulfan were also excluded.
The etiology of CNS complications was determined by clinical history, symptoms, and microbiological, electrophysiological, and radiological characteristics. CNS complications were grouped into 6 categories, namely, calcineurin inhibitor (CNI)-associated neurotoxicity, cerebrovascular events, metabolic encephalopathy (associated with renal failure, liver failure, or electrolyte imbalance), CNS infection, thrombotic microangiopathy (TMA)-associated neurological manifestations, and radiotherapy/chemotherapy toxicity. CNI-associated neurotoxicity was defined as any acute encephalopathy that could be related to the administration of CsA or tacrolimus based on the exclusion of other causes. CNI-associated neurotoxicity was characterized by seizures, changes in the mental status, involuntary movement, or visual disturbance that might be associated with hypertension. The identification of CNI-associated neurotoxicity was supported by the clinical history, electrophysiological, and radiological findings. Trough levels of CsA and tacrolimus at the onset of CNS complications were also reviewed.
3. Conditioning regimen and GVHD prophylaxis
The conditioning regimen for each patient depended on the underlying disease, type of donor and graft, and comorbidities. Myeloablative regimens consisted of total body irradiation (TBI, 6 fractions of 2 Gy each) or busulfan (16 mg/kg); most patients also received cyclophosphamide (120-200 mg/kg). If busulfan was administered, prophylactic phenytoin (5 mg/kg/day) was administered in children older than 10 years. Reduced-intensity regimens were based on fludarabine (150 mg/m2) in variable combinations with busulfan (8 mg/kg), cyclophosphamide (120 mg/kg), low-dose TBI (2 Gy), or antithymocyte globulin (ATG).
GVHD prophylaxis consisted of CsA combined with one of the followings: methotrexate, corticosteroid, or mycophenolate mofetil (MMF). The target serum concentration of CsA was 150-300 ng/mL. Tacrolimus was used for the patients with GVHD who were unsuccessfully controlled with CsA. The target serum concentration for tacrolimus was between 5-15 ng/mL. Acute GVHD was graded according to the standard criteria [
9]. Patients were considered evaluable for acute GVHD, if they survived after engraftment.
4. Statistical analysis
The cumulative probability of developing CNS complications within 6 months after transplant was determined using the Kaplan-Meier method. Univariate analysis was performed to determine the significant risk factors for developing CNS complications. When the number of events was not large enough, the Fisher's exact test was used for categorical variables. Cox proportional hazard model was used on multivariate analysis to determine the impact of the various independent risk factors. P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS (Statistical Package for the Social Science, version 15.0, SPSS Inc, Chicago, IL, USA) and GraphPad Prism (version 5.01, GraphPad Software Inc, La Jolla, CA, USA).
RESULTS
1. Patient characteristics
The clinical characteristics of the 202 patients (128 men, 74 women) are summarized in
Table 1. The median age at transplant was 7.1 years (range, 0.6-18.4 years). Sixty patients (29.7%) received HSCT from matched related donors (MRDs), 9 (4.5%) from mismatched related donors, which included 4 haploidentical mothers, and 133 (65.8%) from unrelated donors. Stem cell sources were bone marrow (BM) in 122 patients (60.4%), peripheral blood (PB) after mobilization with granulocyte colony-stimulating factor in 52 (25.7%), and cord blood stem cells in 28 (13.4%). The median follow-up after transplant was 60 months (range, 7-154 months).
2. CNS complications
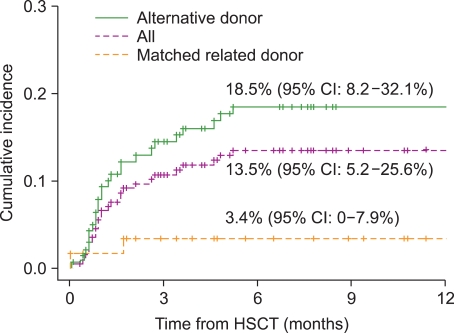
CNS events occurred in 27 children at a median of 31 days after transplantation (range, 0-155 days) (13.5%, [95% confidence interval [CI]: 5.2-25.6]) (
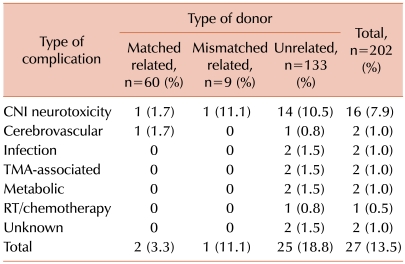
Fig. 1). Neurological symptoms included seizures in 15 patients (56%), altered level of consciousness in 12 (44%), severe headache in 6 (22%), motor weakness in 2 (7%), paresthesia and sensory deficit in 1 (4%), tremor in 1 (4%), and visual disturbance in 1 (4%). The etiologies of CNS events were CNI-associated neurotoxicity in 16 children (59%), cerebrovascular events in 2 (7%), CNS infection in 2 (7%), TMA-associated neurological manifestations in 2 (7%), metabolic encephalopathy in 2 (7%), irradiation/chemotherapy toxicity in 1 (4%), encephalopathy of unknown cause in 1 (4%), and myelopathy of unknown cause in 1 (4%) (
Table 2).
The incidence of CNS complications in the alternative donor group (including mismatched/haploidentical related and unrelated donors) was significantly higher than that in the MRD group (18.5% vs. 3.4%,
P=0.007) (
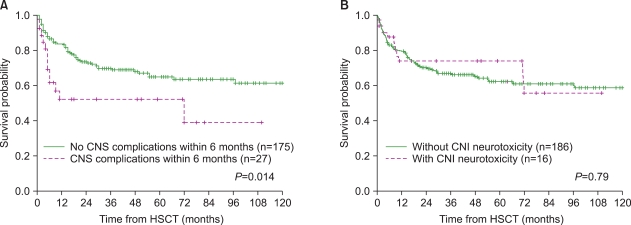
Fig. 1). Of the 27 patients with CNS complications, 14 (52%) died between 4 days and 72 months (median, 4.7 months) after HSCT, and at a median of 35 days (range, 4 days-71 months) following CNS complications. The death of 4 patients was considered a direct result of CNS complications: cerebrovascular events in 2 patients and CNS infection in 2. The remaining 10 patients died of other transplant-related causes: sepsis in 6, veno-occlusive disease in 1, acute GVHD in 1, pulmonary hemorrhage in 1, and sudden asphyxia in 1. Mortality due to CNS complications represented 5.4% of all deaths following HSCT. The 5-year overall survival (OS) rate was significantly lower in patients who developed CNS complications within 6 months of transplant (52.1% vs. 64.9%,
P=0.014). With regard to CNI-associated neurotoxicity, however, the 5-year OS rate was not significantly different between the groups of patients with and without CNI-associated neurotoxicity (74.0% vs. 62.1%,
P=0.79) (
Fig. 2).
3. CNI-associated neurotoxicity
Of the 16 patients with CNI-associated neurotoxicity, 15 patients were alternative donor HSCT recipients (14 unrelated and 1 haploidentical), and 1 patient received the transplant from a MRD. The incidence of CNI-associated neurotoxicity in the unrelated donor HSCT recipients was significantly higher than that in the MRD HSCT recipients (10.5% vs. 1.7%, P=0.007). CsA was a potential etiologic agent in 12 patients and tacrolimus in 4. Twelve out of 16 patients (75.0%) had acute GVHD and, of note, 10 of them were among the 29 patients who had acute GVHD higher than grade 2, and 2 were among the 56 with GVHD grade 1 or 2 (P<0.001).
Neurological symptoms of CNI toxicity were accompanied with hypertension at the time of CNS events in 12 patients (75%). Corticosteroid therapy was used in combination with CNI at the time of CNS events in 13 patients (81%). Neuroimaging was performed in all patients (9 with computed tomography (CT) and magnetic resonance imaging (MRI), 4 with CT only, and 3 with MRI only). MRI revealed typical white-matter lesions with occipital predominance consistent with posterior reversible encephalopathy syndrome (PRES) in 4 patients, focal cortical/subcortical lesions without topical preponderance in 6, signal abnormalities in the prechiasmatic optic nerve in 1, which suggested optic neuropathy, and nonspecific atrophic changes in 1. The CT scans were not informative enough because focal abnormalities that correlated with the MRI findings were only seen in 3 of 13 patients examined by CT, and 5 CT scans did not reveal abnormalities that were detected by MRI.
When symptoms appeared, CsA and tacrolimus levels were within the target range in 10 patients and beyond it in 6. In addition to supportive care, the initial management of CNI-associated neurotoxicity consisted of the change in medication (n=7), dose reduction (n=7), discontinuation for a short period and resumption at a lower dose (n=1), and discontinuation (n=1). None of these patients died of a direct consequence of CNI-associated neurotoxicity. Of the 11 patients whose neuroimaging showed focal abnormalities, 7 patients recovered leaving no neurological sequelae with complete resolution of the focal lesions on MRI, 2 developed intractable seizures even after their MRI abnormalities were resolved, and the remaining 2 died of other transplant-related causes. Of the 5 patients who did not have proven abnormalities on neuroimaging, 2 patients developed Lennox-Gastaut syndrome (1 patient showed bilateral hippocampal atrophy on MRI performed 6.7 years after HSCT, and the other had complications due to hypoxic brain injury after resuscitation from hemorrhagic shock), while the remaining 3 died of other transplant-related causes.
4. Risk factors
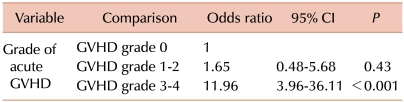
The analysis of the risk factors for developing early CNS complications included gender, age at HSCT, diagnosis, CNS involvement, CNS radiotherapy, type of donor and stem cell source, intensity of the conditioning regimen, treatment with TBI, fludarabine, busulfan, or ATG, GVHD prophylaxis, and occurrence and severity of acute GVHD (
Table 1). In the univariate analysis, the type of donor for HSCT (matched related vs. mismatched related or unrelated,
P=0.01) and the occurrence of acute GVHD (
P<0.001) were associated with an increased risk of CNS complications within 6 months after transplantation. The stem cell source tended to be associated with an increased risk of CNS complications with borderline significance (
P=0.06). When factors with a
P<0.2 were evaluated in the multivariate analysis, grade 3 or 4 of acute GVHD was shown to be an independent prognostic factor for the risk of early CNS complications (odds ratio [OR]=11.96, [95% CI: 3.96-36.11],
P<0.001) (
Table 3).
DISCUSSION
The cumulative incidence of CNS complications within 6 months after HSCT was 13.5% and the mortality rate as a direct result of CNS complications was 5.4% of all deaths following HSCT. This result indicates that CNS complications represent a significant clinical problem among children undergoing allogeneic HSCT. The significant risk factors for early CNS complications were alternative (mainly unrelated) donor transplant, and acute GVHD higher than grade 2. Cord blood as a stem cell source was a risk factor with borderline significance.
Our study focused on clinically significant neurological problems, which required immediate diagnostic effort and treatment. Because of the possible limitations of retrospective studies, it is likely that less severe and other functional problems might be underestimated. In fact, a prospective study that evaluated neurological, neuropsychological, and neuroradiological abnormalities, including clinically less significant problems, reported that 47% of the patients developed new neurological abnormalities after allogeneic HSCT [
10]. Our observation of 13.5% incidence is comparable to other large studies, which reported an incidence of significant neurological complications between 9.7% and 15.8% in pediatric HSCT [
1,
3,
4]. In contrast to observational studies, an autopsy study revealed that neuropathological findings were detected in 90% of autopsies performed in bone marrow transplant (BMT) patients, which suggests that most patients with life-threatening illness will experience neurological events [
8].
Allogeneic transplants (in studies including both autologous and allogeneic transplantation), acute GVHD > grade 2, and unrelated donor transplant have been identified as significant risk factors for neurological complications in most of the previous studies. The use of TBI [
3,
5], female gender [
5], electrolyte imbalance [
11], pretransplant viral status [
11], and diagnosis of acute myeloid leukemia (AML) [
4] or chronic myelogenous leukemia [
12] have been inconsistently described as possible risk factors by various authors. We found that alternative (mainly unrelated) donor transplants and grade 3-4 acute GVHD were significant risk factors related to CNS complications in allogeneic HSCT recipients, which confirms the observations made in other studies. Multivariate analysis showed that severe acute GHVD was an independent risk factor for the occurrence of CNS complications. In patients undergoing allogeneic transplantation, the use of immunosuppressive agents, such as CsA and tacrolimus, is an essential component in the control of GVHD. Patients who experience severe acute GVHD are predisposed to CNI-associated neurotoxicity because they are usually managed with aggressive immunosuppression therapy. In addition, corticosteroids used during the treatment of GVHD may contribute to the development of neurological events [
13]. Moreover, it has been reported that patients with severe GVHD tend to experience not only CNS complications associated with immunosuppressive drugs but also CNS infections or TMA-associated neurological events due to immunosuppression [
1,
14].
Alternative donor transplantation, especially unrelated donor transplantation, could have an impact on the development of CNS complications due to its association with a high incidence of GVHD. Although the graft source has been inconsistently reported as a risk factor for the development of CNS complications [
4], our study showed that cord blood as a graft source was a significant risk factor for CNS complications compared to a BM stem cell graft (25% vs. 9.8%). Cord blood recipients are more susceptible to infectious complications and likely to be exposed to the risk of hemorrhagic complications due to delayed engraftment.
CNI- or CsA-associated neurotoxicity has been consistently reported as the most frequent cause of neurological complications after transplantation in previous studies [
2-
5]. In our study, CNI-associated neurotoxicity was the most common neurological event with an incidence of 7.9% and 10.5% among all enrolled patients and among 133 unrelated donor transplant recipients, respectively. Patients with CNI-associated neurotoxicity may have variable white-matter lesions with or without cortical involvement on MRI [
15]. The previous studies reported that the incidence of PRES ranged between 5.9% and 7.2% in allogeneic HSCT patients receiving CNIs, which seems higher than our observation [
5,
16,
17]. These results imply that more thorough neuroradiological evaluations could more effectively detect events of PRES. Although marked abnormalities can be detected by CT, CT has a limited role in the detection of cerebral parenchymal lesions associated with CNI neurotoxicity. Thus, MRI should be performed early in the diagnostic evaluation of patients on CNIs who develop significant CNS events. In particular, 1 patient in our series developed optic neuropathy associated with CsA, which has been rarely reported as a neurotoxic side effect of CsA [
18,
19]. Thus, optic side effects as well as PRES should be ruled out when patients during CsA therapy develop visual disturbance after HSCT.
Patients who develop seizures or encephalopathic features tend to have elevated blood pressure (BP) at the time of the CNS events. In this context, CNI-associated neurotoxicity could encompass patients who were historically thought to have hypertensive encephalopathy [
20]. The serum levels of CsA or tacrolimus were slightly elevated in only 6 of 16 patients with CNI-related toxicity at the time of CNS events, and did not increase far above the upper limit in any patient. It was suggested that genetic differences in the CsA metabolism might be related to CsA toxicity at the therapeutic blood levels in a case report [
21]. In addition, the lack of a direct correlation between CsA levels and CsA-associated neurotoxicity was previously described [
15,
22]. These findings emphasize that therapeutic drug monitoring cannot predict all adverse events associated with CNIs. Thus, recognition of prodromal signs such as tremulousness or headache, meticulous control of the BP, and correction of the electrolyte imbalance are important for the early detection and prevention of CNI-associated toxicity.
CNI-associated toxicity is known to be reversible after withdrawal of the drug [
23]. However, irreversible cases have been reported in a few instances [
24]. It is important to note that 4 patients developed intractable seizures later during the long-term follow-up despite the resolution of focal white-matter lesions in 2 patients and the absence of documented lesion at initial presentation in the other 2. This result suggests that long-term results of CNI-associated toxicity may not always be reversible, regardless of the resolution of the initial lesions on neuroimaging. In particular, 1 patient showed bilateral hippocampal atrophy on the follow-up MRI. Faraci et al. also reported 3 patients who developed intractable seizures with mesial temporal sclerosis on MRI after CsA-associated neurotoxicity [
3]. This late complication might be associated with an unrecognized acute lesion on the hippocampus, since acute hippocampal lesions documented on MRI were reported as CNI-mediated neurotoxicity [
25].
Our data demonstrated a significantly reduced survival rate for patients with CNS complications. However, when the comparison was done between patients with and without CNI-associated toxicity, significant differences in the OS were not observed. These findings suggest that CNS complications, excluding fatal complications, such as cerebrovascular event and CNS infection, are not associated with long-term survival. Iguchi et al. also reported that significant differences in the long-term survival were not observed between children with and without neurologic complications, whereas adverse neurological events contributed to early death [
1]. In contrast, Siegal et al. reported significantly reduced survival in patients with CNS complications as well as in those with PRES in their adult series [
5]. GVHD has been reported to represent the primary cause of death following CNI-associated neurotoxicity in the previous adult studies [
5,
17]. However, our report showed that only 1 patient with CNI-associated toxicity died of a direct consequence of acute GVHD. The discrepancy between pediatric and adult studies might reflect differences in the susceptibility to GVHD between children and adults. Since discontinuation of CNI administration may cause poor prevention and management of GVHD resulting in significant mortality due to GVHD, decisions on the changes in the immunosuppressive agent should be made carefully in order to balance the control of GVHD and the management of CNI-associated neurotoxicity.
In conclusion, CNI-associated toxicity was the main cause of neurotoxicity in children receiving allogeneic HSCT, followed by cerebrovascular events, infection, metabolic encephalopathy, and TMA-associated neurological abnormalities. These results imply that more attention should be paid to the recognition of the development of CNS complications for recipients of alternative donor transplants (especially unrelated donor) and patients with severe acute GVHD, who are at a significantly increased risk of CNS complication. Immediate and comprehensive diagnostic work-up, especially neuroimaging, is required to detect neurological events in any patients presenting with neurological symptoms.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download