COMMON PLASMA PRODUCTS
There are numerous plasma products listed in the circular of information for the use of human blood and blood components, a joint effort of the AABB (formerly known as the American Association of Blood Banks), the America's Blood Centers, the FDA and the American Red Cross [
1]. This short review will focus on the most commonly used plasma components, fresh frozen plasma (FFP), thawed plasma (TP), and plasma frozen within 24 hours after phlebotomy (FP24). As the clotting factors in these products are nearly equivalent, the generic term "plasma" will be used throughout this review.
FFP is prepared from whole blood donations or is collected by plasmapheresis, and in 2006 it accounted for about 77% of all plasma transfused in the USA [
2]. To be labeled as FFP, the plasma must be frozen at -18℃ or less within 8 hours of collection [
3]. By definition FFP contains 1 IU/mL of all clotting factors. There is, however, variability in coagulation factor levels as demonstrated in a study looking at the INR of 20 units of FFP; the mean INR was 1.1 while the range was 0.9-1.3 [
4]. This could have ramifications if plasma units with relatively higher INRs are used to try to reverse a recipient's mildly elevated INR (see below).
FFP is stored at -18℃ or less for up to 1 year [
3]. It is thawed in a 30-37℃ water bath generally when an order for transfusion is received by the blood bank and this step normally takes 20-25 minutes. Once thawed, the FFP can be kept at 1-6℃ for up to 24 hours without significant loss of clotting factors. If the FFP is not used within 24 hours, it must either be discarded or relabeled as thawed plasma (TP).
TP is the product that can result if liquid plasma is maintained at 1-6℃ for more than 1 day; once FFP has been thawed and refrigerated for more than 24 hours, it can no longer be labeled as "fresh frozen" plasma. Instead the thawed unit can be relabeled as TP, and it can continue to be stored in the liquid state at 1-6℃ for up to 4 more days [
3]. A recent study demonstrated that for both leukoreduced aphersis or whole blood plasma stored in the liquid state between 2-6℃, the level of factor VIII decreased to approximately 0.40 IU/mL at 28 days of storage [
5]. Other studies of TP prepared from FFP demonstrated approximately 14-55% decreases in factor VIII levels at day 5 of storage compared to day 1 [
6,
7], but these residual levels, including that of ADAMTS-13 after 5 days of storage, were still well within the therapeutic range [
8-
10]. The decreases in factor V levels were smaller [
6,
8] even after 28 days of storage [
5]. TP has the advantage of already being in a liquid state thus eliminating the time delay caused by thawing the frozen FFP, and it effectively extends the shelf-life of a unit of plasma that would otherwise have been discarded, thereby reducing wastage of a limited resource.
In an effort to reduce the risk of transfusion related acute lung injury (TRALI) from plasma components, many blood centers have limited the collection of plasma from female donors due to their propensity for developing anti-HLA alloimmunization after pregnancy [
11]. To avoid a shortfall in the plasma supply, there is increasing reliance on the production of plasma from male whole blood donations that have been stored between 8-24 hours prior to freezing. This product is known as plasma frozen within 24 hours after phlebotomy (FP24) and in 2006 it accounted for approximately 15% of all the plasma transfused in the USA [
2]. The levels of most clotting factors are not significantly diminished compared to regular FFP at the time of thawing [
7,
12-
15], and recently 2 studies have demonstrated that the levels of clotting factors remain hemostatic during 5 days of refrigerated storage of TP prepared from FP24 [
16,
17]. In one study the levels of V and VIII declined to means of 59 and 48 IU/dL [
17], respectively, on storage day 5, and in the other study the level of protein S (PS) was slightly below the reference range by day 5 [
16]. These minor changes are unlikely to be clinically significant. Recently Alhumaidan et al. demonstrated that FP24 could be prepared from either platelet rich plasma or whole blood donations and stored for up to 7 days at refrigerator temperatures with maintenance of clotting factors at clinically adequate levels [
18]. It should be noted, however, that the only currently AABB/FDA approved starting material for TP is FFP [
3]. Furthermore, neither FP24 nor the TP products have been directly compared to FFP in terms of their efficacy in reversing coagulopathies or arresting coagulopathic bleeding, however based on the well preserved coagulation factors there is no a priori reason why these products would be inferior to FFP.
Go to :

USE OF PLASMA
The use of plasma in the USA is growing. In 2006 approximately 4 million units were transfused [
2], which is several orders of magnitude higher than in several other developed countries [
20]. The indications for plasma transfusion include reversal of a significant coagulopathy in a bleeding patient or one who is about to undergo a surgical procedure, bleeding in the setting of multiple factor deficiencies, or in the rare patient with a deficiency of a factor for which there is no viral-inactivated/recombinant concentrate available. Neither its use as a replacement fluid for therapeutic aphersis in thrombotic thrombocytopenic purpura (TTP) patients, nor the pharmacological procoagulant agents (such as rfVIIa) that might also be used to reverse a significant coagulopathy will be discussed in this report. The use of plasma as part of fixed ratio RBC: plasma protocols for the resuscitation of trauma patients is controversial and is extensively reviewed in reference [
21]. Often times the question facing the clinician when trying to decide whether to transfuse plasma is: When is a coagulopathy significant enough for the benefits of plasma transfusion to outweigh its potential adverse events such as TRALI and volume overload?
To answer this question, 2 important meta-analyses have been performed. Segal and Dzik analyzed 25 studies of variably coagulopathic patients undergoing a variety of minor procedures including liver biopsy, kidney biopsy, central vein cannulation, paracentesis and others and asked the question, does the peri-operative PT/INR predict the risk of major bleeding during these procedures [
22]? The vast majority of the reports included in this meta-analysis were observational studies, and only 1 was a clinical trial. The authors concluded that the strongest evidence suggesting that the pre-procedure INR does not likely predict the bleeding risk lies with central vein cannulation, although just how coagulopathic patients can be and still tolerate the procedure safely has not been elucidated. As for the literature on the other procedures, the variability in study size and quality makes drawing firm conclusions about the bleeding risk difficult. In 14/25 of the studies included in the meta-analysis, a control group of patients with normal laboratory parameters of coagulation were also included in the report. In these studies, the risk of bleeding between the 2 groups of patients undergoing the same procedure could be estimated [
22]. Although the confidence intervals of some of these comparisons were relatively large due to the small number of patients in these studies, there was no significant difference in the risk of major bleeding between the patients who underwent these varied procedures with and without coagulopathies. While further study is required, especially for coagulopathic patients undergoing kidney biopsy, overall it would appear that patients with mild coagulopathies undergoing various surgical procedures might not require normalization of their laboratory coagulation parameters with plasma to reduce their risk of bleeding. For further discussion of why the PT/INR does not necessarily predict the risk of peri-operative bleeding, see reference [
23].
The second meta-analysis can shed some light on the question, if plasma is administered to peri-surgical patients, does it have a beneficial effect in reducing transfusion requirements or surgical blood loss? Stanworth and colleagues searched various medical publication databases looking exclusively for randomized controlled trials (RCT) where FFP was the therapeutic intervention [
24]. While 57 such trials were identified, 19 were focused on surgical or potentially surgical patients; there were 11 studies based on cardiovascular surgery in children and adults, 3 studies on liver disease with or without GI bleeding, and 1 study each on warfarin reversal with intracerebral hemorrhage, massive transfusion, hip surgery, hysterectomy, and renal transplantation. In only 12/19 of these studies (10 cardiovascular surgery, 1 liver disease, and the lone study on hysterectomy) did the patients in the control arm receive either no FFP or a colloid volume expander, thus allowing a true evaluation of the effect of the FFP administration. Most of these studies concluded that FFP administration did not reduce blood loss or transfusion requirements [
24].
To explain why prophylactic plasma administration does not reduce peri-operative bleeding, consider a study of 22 non-trauma patients who received a total of 68 units of FFP (500 mL units) [
4]. On average each recipient received approximately 2 units of FFP, and this dose was >10 mL/kg for each recipient. The average pre-transfusion INR was 1.37 (range 1.1-1.6), and the average decrease in INR was a clinically insignificant 0.03 per unit of FFP transfused.
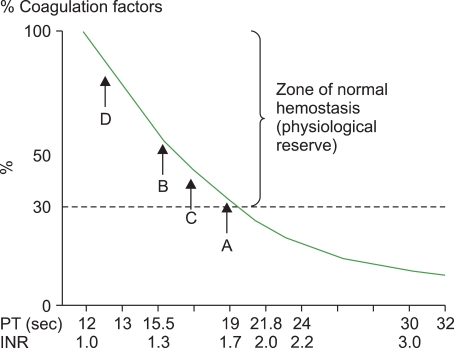
Fig. 1 demonstrates a theoretical relationship between PT/INR and the concentration of clotting factors; a recipient with INR=1.37 would likely have clotting factor concentrations in excess of 50% of normal (
Fig. 1, point B). From the experience with patients with single clotting factor deficiencies such as hemophilia A and B, levels of clotting factors >30% are sufficient for normal hemostasis. Thus the recipients with an INR=1.37 were unlikely to have bled excessively even in the absence of plasma transfusion, and the slight reduction in INR did not confer any improvement in the recipient's hemostatic potential. Furthermore, given that some FFP units can have INRs approaching that of these recipient's [
4], it is not surprising that the decreases in the post-transfusion INRs were quite modest.
Abdel-Wahab and colleagues studied FFP recipients from a wide variety of hospital wards, and with and an assortment of clinical diagnoses [
25]. In this retrospective study, 324 FFP units were transfused to 121 recipients who had relatively low pre-transfusion INRs, 1.1-1.85, and who had post-transfusion INRs performed within 8 hours of the transfusion (the approximate therapeutic duration of plasma). Once again, only small median reductions in the post-transfusion PT and INR were seen; 0.20 seconds and 0.07, respectively. Only 1 of the 324 recipients completely corrected their PT and INR, and only 15% of patients corrected their PT/INR to 50% of normal. It is not surprising that patients with lower INRs (1.1-1.5) were no more likely to correct their coagulation parameters than those with slightly higher INRs (1.5-1.85), and these authors did not find a correlation between the magnitude of the change in the INR and the dose of FFP administered; recipients of 1 unit of FFP were no more likely to correct their INR by 50% than those who received 2 units [
25].
The latter finding can be explained by considering that 1 unit of FFP (approximately 225 mL), when administered to a 70 kg recipient, translates into a dose of 3.2 mL/kg. Receipt of 2 units of FFP by a 70 kg recipient would amount to a dose of 6.4 mL/kg - both doses are significantly below the recommended 10-15 mL/kg of plasma to correct a coagulopathy. Furthermore, each recipient in this study received on average 2.7 units of FFP, and assuming each recipient weighed 70 kg, the average dose of FFP would only have been 8.7 mL/kg [
25]. Thus the low rate of correction could be attributable to the small volume of FFP transfused. Additionally, consider that the plasma volume of a 70 kg recipient with a Hct of 0.40 is approximately 3,000 mL. Transfusing 450 mL of plasma (2 regular units) would increase the concentration of clotting factors by 15%; according to
Fig. 1, a 15% increase in clotting factors in a recipient with an INR of 1.5 would only amount to a small decrease in their INR (
Fig. 1, point C). A recipient with an INR=1.5 still has a considerable reserve of clotting factors and a slight increase in their concentration is unlikely to be important for hemostasis. This might also explain the popular perception of the success of prophylactically administering plasma to recipients with modestly elevated INRs; when these patients tolerate the surgical procedure without excessive bleeding, this positive outcome is attributed to the administration of the plasma. In reality, these recipients were unlikely to have had a coagulopthic bleed owing to their significant reserve of clotting factors (even with their slightly elevated INR). Another study of plasma recipients in Canada also found a minimal INR response when mildly coagulopathic patients were transfused with small quantities of FFP [
26].
A study of patients with liver cirrhosis who were transfused with FFP was performed in 2 parts: a retrospective review of the charts of 80 patients who received FFP to reverse a PT prolonged by >3 seconds, and a prospective analysis of 20 patients who received FFP with the same laboratory abnormality [
27]. The indications for FFP ranged from pre-procedure prophylaxis, acute bleeding, or prophylactic reversal of a prolonged PT in non-bleeding recipients. The etiology of the cirrhosis in the majority of these patients was either alcohol use or a combination of Hepatitis C and alcohol use. These investigators found only a small number of FFP recipients who corrected their PT to within 3 seconds of normal after FFP transfusion (12/100). Interestingly, the 2 recipients with the highest pre-transfusion PTs who received 6 units of (PT=23.8s and 22.9s) FFP demonstrated on average a 9.75s reduction in their PT after transfusion. The 2 recipients with the lowest PT who also received 6 units of FFP (both had PT=15.5s) both demonstrated only a 0.4s reduction after transfusion [
27]. While the authors did not convert PT into INR, a PT=15.5s roughly translates into an INR of approximately 1.3-1.5 depending on the sensitivity of the PT reagent, once again indicating that in patients with a relatively mild coagulopathy, minimal correction is expected after plasma transfusion.
It might be hypothesized that the low rates of PT/INR correction after plasma infusion is related to the fact that the patients in these clinical studies have been quite sick. However, a different type of study evaluating the recovery of factor VII in normal FFP compared to a pathogen inactivated (S-59+UVA light) form of FFP in healthy volunteers showed a similar magnitude of PT correction as the earlier studies [
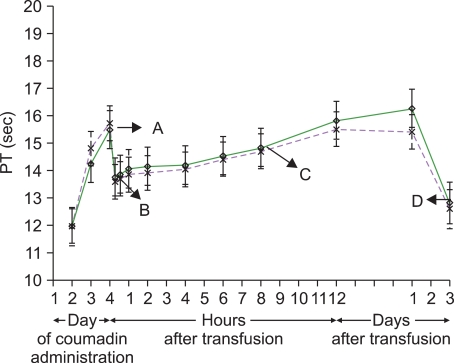
19]. In this study, 27 healthy volunteers donated approximately 2.5 L of autologous plasma over time and were then administered warfarin to deplete their levels of vitamin K-dependent clotting factors, which includes factor VII. After they were anticoagulated to INRs between 1.5-2.0 they were reinfused with 1 L of either their unaltered plasma or their plasma after it had undergone pathogen inactivation after one final dose of warfarin (
Fig. 2, point A). This dose of plasma was on average 12 mL/kg for all study participants. The investigators then measured a series of pharmacokinetic parameters of factor VII, along with a PT. Immediately prior to FFP administration, the average PT was 15 seconds, and within 1 hour after FFP administration, it had dropped to approximately 13 seconds (
Fig. 2, point B). This 2 second decrease in PT represents only about 50% correction compared to the average pre-anticoagulation baseline PT of 11.5 seconds. At the end of the study period, all volunteers were given oral vitamin K which resulted in a complete reversal of their PT prolongation which should serve as a reminder that in situations when reversal of a coagulopathy is not urgent, non-human source medications such as vitamin K could be employed (
Fig. 2, point D) [
19]. Interestingly, while only 15% of the sick patients in the study by Abdel-Wahab et al. corrected their PT/INR by 50% [
25], the average PT correction by the healthy volunteers in this study was 50% [
19]. This 50% correction of the PT could have been predicted from
Fig. 1: Again, if the average plasma volume of the healthy recipients was 3,000 mL, and they received 1,000 mL of FFP, then their clotting factors should have been increased by 33%. A 33% increase in clotting factors is consistent with a decrease of about 2 seconds (from 15 to 13 seconds) (
Fig. 1, from point B to D). While these healthy volunteers [
19] received on average about 3 mL/kg more FFP than those reported by Abdel-Wahab et al. [
25], perhaps other factors are involved in determining a recipient's propensity to correct their PT/INR after plasma infusion. Furthermore, Holland et al. demonstrated that some patients with mildly prolonged INRs (1.3-1.6) will effect some degree of correction without plasma therapy by simply having their underlying diseases treated and receiving supportive care [
28].
Another way to consider the effect of transfusing plasma to patients with an elevated INR is to remember that the response of a patient who is otherwise stable (i.e., not bleeding) depends mostly on the pretransfusion INR. This is depicted in
Fig. 3 which is based on an equation derived from a clinical study of 140 adults who received jumbo apheresis plasma units (500 mL) [
28]. These patients were not involved in trauma, not in the operating room, did not have DIC and were not treated with prothrombin complex concentrates (PCC). Clearly the largest reductions in the INR are predicted to occur in recipients with the highest starting INRs; in theory as the INR falls below 1.5, continued transfusion with plasma is predicted to effect only a small and clinically insignificant change in the INR. At an INR≤1.5 the recipient is being exposed to all the adverse risks of plasma therapy without any clinical benefit.
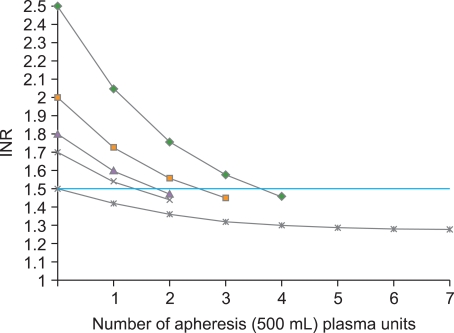 | Fig. 3Theoretical response to plasma transfusion based on a formula derived from a clinical study of 140 adult plasma recipients [ 28]. The main predictor of the response to plasma transfusion is the pretransfusion INR. 
|
Perhaps the currently employed dose of plasma to reverse coagulopathies is insufficient to effect a significant reduction in the PT/INR. A small Welsh study extensively evaluated the laboratory parameters of hemostasis including factor levels, PT and PTT in ICU patients before and after receiving a median dose of either 12.2 mL/kg (n=10) or 33.5 mL/kg (n=12) of FFP [
29]. They found much larger increases in the post-transfusion factor levels and decreases in the PT and PTT (especially the PTT) in the recipients of the higher dose of FFP. While this study did not investigate the clinical impact of higher levels of clotting factors or lower PT and PTT values on these recipients, it is possible that the currently recommended dose of 10-15 mL/kg is insufficient to reverse a coagulopathy [
29].
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download