Abstract
A subgroup of acute leukemia with morphology resembling acute promyelocytic leukemia (APL) shows variant translocations involving RARA and has a different morphology from that of classical APL. The variant APL with t(11;17)(q23;q12); ZBTB16-RARA subgroup has been reported to have leukemic cells with regular nuclei, many granules, absence of Auer rods, an increased number of Pelgeroid neutrophils, strong myeloperoxidase (MPO) activity, and all-trans-retinoic-acid (ATRA) resistance. Here, we report a case of variant APL with t(11;17)(q23;q12); ZBTB16-RARA showing typical morphological features of classical APL, including numerous Auer rods and faggot cells. The leukemic cells expressed CD13, CD33, CD117, human leukocyte antigen (HLA)-DR, and cytoplasmic-MPO on the immunophenotyping study. The diagnosis was confirmed by cytogenetic and molecular studies. To distinguish variant APL cases from classical APL cases, regardless of whether morphologically the findings are consistent with those of classical APL, combining morphologic, immunophenotypic, cytogenetic, and molecular studies before chemotherapy is very important.
Acute promyelocytic leukemia (APL) with t(15;17)(q22;q12); PML-RARA is characterized by the presence of promyelocytes and multiple Auer rods, hemorrhagic tendency, and PML/RARA fusion mRNA [1-4]. A subgroup of acute leukemia that has a similar morphology as APL shows variant translocations involving RARA. These variant fusion partners include ZBTB16 at 11q23, NUMA1 at 11q13, NPM1 at 5q35, and STAT5B at 17q11.2 [5-8]. Some of the variant APL with t(11;17)(q23;q21) cases that are associated with the ZBTB16-RARA fusion gene have been reported as being resistant to all-trans-retinoic acid (ATRA) [9]. Therefore, differential diagnosis of variant APL with t(11;17)(q23;q12) from classical APL with t(15;17)(q22;q12); PML-RARA is very important. Variant APL cases usually show different morphological characteristics. The variant APL with t(11;17) (q23;q12); ZBTB16-RARA subgroup has been reported to have leukemic cells with regular nuclei, many granules, absence of Auer rods, an increased number of Pelgeroid neutrophils, and strong myeloperoxidase (MPO) activity [10]. Therefore, whe n leukemic cells show typical morphology of classical APL with t(15;17)(q22;q12); PML-RARA, the possibility of variant APL is very low. We report a case of variant APL with the t(11;17)(q23;q12); ZBTB16-RARA showing typical morphology of classical APL.
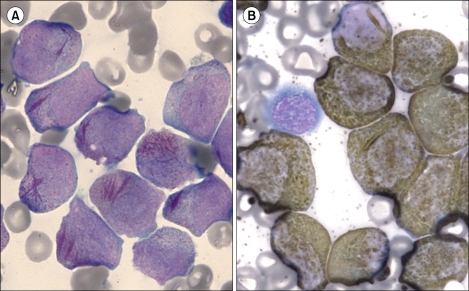
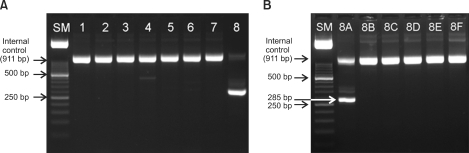
In January 2010, a 52-year-old man was referred to our hospital because of pancytopenia. Complete blood count (CBC) showed pancytopenia with white blood cells, 1,620/µL (segment neutrophils, 12.3%; lymphocytes, 60.5%; monocytes, 27.2%; eosinophils, <1%; basophils, <1%; leukemic cells, 8%); hemoglobin, 8.8 g/dL; and platelets, 98,000/µL. Bone marrow biopsy revealed about 100% cellularity, and that 80% of the nucleated elements were leukemic cells. The leukemic cells showed medium to large size, irregular shape, finely chromatinized nuclei with distinct nucleoli, and moderate amount of blue cytoplasm with azurophilic granules. The leukemic cells showed multiple Auer rods and frequently showed faggot cells (Fig. 1A). They were strongly positive to MPO stain (Fig. 1B). The leukemic cells expressed CD13, CD33, CD117, human leukocyte antigen (HLA)-DR, and cytoplasmic-MPO, as revealed by the immunophenotyping study. ZBTB16-RARA translocation was detected on the leukemia gene screening test by reverse transcription-nested polymerase chain reaction (Fig. 2; Hemavision, DNA technology, Denmark). Cytogenetic study revealed t(11;17)(q23;q21) (Fig. 3). The patient is being treated with chemotherapy without ATRA.
Most APLs show typical morphology, including irregular and variable nuclei that are often kidney-shaped or bilobed; cytoplasm containing densely packed or even coalescent large granules that stain bright pink, red, or purple in Romanowsky stains; and multiple Auer rods [1-4, 10]. Therefore, APL can be easily diagnosed at the time of morphologic evaluation. However, variant APL t(11;17)(q23;q21) has been reported to have atypical APL morphology, and Auer rods are seldom observed [9, 10]. Absence of Auer rods or faggot cells is especially considered as a characteristic morphologic feature of this subtype [9, 10]. In variant APLs, including variant RARA translocation, the diagnosis can be made by chromosome analysis or molecular study [11-13]. The results may show the typical karyotype t(15;17)(q22;q12); PML-RARA or the variant RARA translocation [11-13]. However, in our case, the typical morphology of classical APL, including multiple Auer rods in numerous leukemic cells and frequent faggot cells, was observed, and the karyotype and molecular studies showed t(11;17)(q23;q12) and ZBTB16-RARA fusion. At the time of morphologic evaluation, our case showed findings consistent with those of APL with t(15;17)(q22;q12), before chromosome analysis and molecular study were performed. The typical immunophenotype of APL has been reported to be distinctive unlike those in other acute myeloid leukemia (AML) cases. The leukemic cells in the former express myeloid antigens such as CD13 and CD33 but show weak or negative reaction to CD34 and HLA-DR [14]. Some of the microgranular variant APL cases express CD34 and/or HLA-DR [15]. However, variant APL with t(11;17)(q23;q12); ZBTB16-RARA subgroup has been reported to show the same immunophenotype as that in classical APL [10]. Interestingly, in our case, the leukemic cells expressed HLA-DR in addition to CD13, CD33, and CD117, unlike other cases that have previously been reported [10]. The variant APL with t(11;17)(q23;q12); ZBTB16-RARA subgroup usually shows resistance to ATRA therapy [9]. Therefore, our patient is being treated with alkylating agents rather than the classical APL regimen that includes ATRA. The present study emphasizes the importance of combining morphologic, immunophenotypic, cytogenetic, and molecular studies to distinguish variant APL cases from classical APL cases before initiating chemotherapy, regardless of whether the morphological study reveals findings consistent with those of classical APL.
References
1. Stone RM, Mayer RJ. The unique aspects of acute promyelocytic leukemia. J Clin Oncol. 1990; 8:1913–1921. PMID: 2230879.

2. Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998; 91:3093–3102. PMID: 9558362.

3. Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999; 340:994–1004. PMID: 10099141.

4. Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976; 33:451–458. PMID: 188440.

5. Chen Z, Brand NJ, Chen A, et al. Fusion between a novel Krüppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993; 12:1161–1167. PMID: 8384553.

6. Corey SJ, Locker J, Oliveri DR, et al. A non-classical translocation involving 17q12 (retinoic acid receptor alpha) in acute promyelocytic leukemia (APML) with atypical features. Leukemia. 1994; 8:1350–1353. PMID: 8057672.
7. Hummel JL, Wells RA, Dubé ID, Licht JD, Kamel-Reid S. Deregulation of NPM and PLZF in a variant t(5;17) case of acute promyelocytic leukemia. Oncogene. 1999; 18:633–641. PMID: 9989813.

8. Wells RA, Hummel JL, De Koven A, et al. A new variant translocation in acute promyelocytic leukaemia: molecular characterization and clinical correlation. Leukemia. 1996; 10:735–740. PMID: 8618456.
9. Licht JD, Chomienne C, Goy A, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17). Blood. 1995; 85:1083–1094. PMID: 7849296.

10. Sainty D, Liso V, Cantù-Rajnoldi A, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. group français de cytogénétique hématologique, UK cancer cytogenetics group and BIOMED 1 European coomunity-concerted acion "molecular cytogenetic diagnosis in haematological malignancies. Blood. 2000; 96:1287–1296. PMID: 10942370.
11. McKenna RW, Parkin J, Bloomfield CD, Sundberg RD, Brunning RD. Acute promyelocytic leukaemia: a study of 39 cases with identification of a hyperbasophilic microgranular variant. Br J Haematol. 1982; 50:201–214. PMID: 6949609.

12. Aventin A, Mateu R, Martino R, Colomer D, Bordes R. A case of cryptic acute promyelocytic leukemia. Leukemia. 1998; 12:1490–1506. PMID: 9737701.

13. Neame PB, Soamboonsrup P, Leber B, et al. Morphology of acute promyelocytic leukemia with cytogenetic or molecular evidence for the diagnosis: characterization of additional microgranular variants. Am J Hematol. 1997; 56:131–142. PMID: 9371524.

14. Paietta E, Andersen J, Gallagher R, et al. The immunophenotype of acute promyelocytic leukemia (APL): an ECOG study. Leukemia. 1994; 8:1108–1112. PMID: 8035602.
15. Guglielmi C, Martelli MP, Diverio D, et al. Immunophenotype of adult and childhood acute promyelocytic leukaemia: correlation with morphology, type of PML gene breakpoint and clinical outcome. A cooperative Italian study on 196 cases. Br J Haematol. 1998; 102:1035–1041. PMID: 9734655.

Fig. 1
Bone marrow aspirates showing typical acute promyelocytic leukemia morphology, including numerous leukemic promyelocytes with faggot cells (A) Wright stain; ×1,000 and strong myeloperoxidase activity (B) myeloperoxidase stain; ×1,000).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download