Abstract
Background
Syndecan-1 is a heparan sulfate proteoglycan expressed on plasma cells, especially myeloma cells, and can exist in serum as soluble syndecan-1 after shedding from the cell surface. Soluble syndecan-1 has been suggested to promote myeloma cell growth and to be an independent prognostic factor for multiple myeloma. We aimed to evaluate the effect of soluble syndecan-1 levels at the time of diagnosis and during therapy on therapeutic response and prognosis for patients with multiple myeloma.
Methods
We analyzed soluble syndecan-1 levels in 28 patients with multiple myeloma and 50 normal controls, and compared its levels with Durie-Salmon stage and other markers of myeloma. In addition, we evaluated the therapeutic response and determined the 3-year survival rates of these patients.
Results
We observed that the median soluble syndecan-1 level in myeloma patients was higher than that in the normal controls (P <0.0001), and the soluble syndecan-1 levels in 21 (75%) patients were higher than the cut-off level (162 ng/mL). Soluble syndecan-1 levels correlated with disease stage, percentage of plasma cells in the bone marrow, β2 microglobulin level, serum M-component concentration, and creatinine level. The baseline levels of soluble syndecan-1 at the time of diagnosis in the patients who responded to chemotherapy were lower than those in the non-responders (P=0.04); however, the baseline level was not a significant predictor of therapeutic response. The 3-year overall survival rate of the patients with high soluble syndecan-1 levels at the time of diagnosis and 6 months after chemotherapy was lower than the corresponding survival rates of the patients with low levels of soluble syndecan-1; however, the overall survival rate was not statistically significant.
Multiple myeloma (MM) is characterized by the proliferation of clonal plasma cells in the bone marrow (BM) and production of monoclonal immunoglobulins. Syndecan-1 (CD138) is a heparan sulfate-bearing proteoglycan expressed by both normal and myeloma plasma cells [1]. Syndecan-1 mediates cell-cell and cell-matrix adhesion of myeloma cells and inhibits the invasion of myeloma cells invasion into type I collagen [2, 3]. Therefore, the shedding of syndecan-1 from the cell surface may contribute to cell proliferation and dissemination. Syndecan-1 is cleaved from the surface of myeloma cells by metalloproteinases and can exist in serum as soluble form [4]. Patients with MM have been reported to have high serum levels of soluble syndecan-1; these high levels can be attributed to a high percentage of plasma cells in the BM and high β2-microglobulin levels [5]. Although soluble syndecan-1 level has been shown to be an independent negative prognostic factor [5, 6], no study has evaluated the diagnostic and prognostic value of soluble syndecan-1 in Korean MM patients. The purpose of this study was to investigate the discriminatory value of syndecan-1 by measuring the levels of soluble syndecan-1 in myeloma patients and normal controls, and to analyze the effect of soluble syndecan-1 levels on the prognosis for patients with multiple myeloma.
We investigated 28 patients (16 men and 12 women; mean age, 64.6±8.8 years; range, 42-75 years) who were newly diagnosed with MM between March 2005 and December 2007, and monitored these patients until March 2009. Peripheral blood and BM aspirates were obtained from the patients at the time of diagnosis; peripheral blood was also obtained 6 months after the initiation of chemotherapy. The blood and BM aspirates were centrifuged, and the serum samples thus obtained were stored at -70℃ until subsequent analysis. The following variables were investigated at the time of diagnosis: percentage of plasma cell in the BM; serum levels of β2 microglobulin; serum M-protein concentration; types of immunoglobulin (Ig) present; serum levels of calcium, creatinine, and lactate dehydrogenase (LD); and free κ/λ light chain ratio. Furthermore, we performed chromosomal analyses of BM specimens. The patients with MM were grouped on the basis of MM stages determined using the Durie-Salmon staging system [7]. Serum samples obtained from 50 healthy individuals (mean age, 60.8±9.1 years; range, 45-76 years, men:women ratio=29:21) were used as normal controls.
Seven MM patients received vincristine, adriamycin, and dexamethasone (VAD) therapy, while 21 patients received cyclophosphamide and prednisolone (CP) therapy. During the follow-up period, 1 MM patient received thalidomide, and 2 patients received thalidomide and bortezomib. Two MM patients underwent stem cell transplantation. Therapeutic response was defined as at least a 50% reduction in serum M-protein concentration at 6 months after the initiation of chemotherapy [8], and the patients were further classified as responders and non-responders according to their therapeutic response. Survival was measured from diagnosis to death or last follow-up.
The concentration of soluble syndecan-1 was measured using a commercially available human syndecan-1 enzyme-linked immunosorbent assay kit (Diaclone Research, Besancon, France). Baseline levels of soluble syndecan-1 were determined before treatment, and follow-up levels were measured 6 months after the initiation of chemotherapy. Briefly, 100 µL of serums and standards and 50 µL of diluted biotinylated antibody were added into pre-coated wells and incubated for 1 h at room temperature (RT). After 3 washes, 100 µL of horseradish-peroxidase-streptavidin conjugate was added, and the plate was incubated for 30 min at RT. After washing, 100 µL of substrate was added, and the color was allowed to develop for 15 min. The reaction was stopped with H2SO4, and the absorbance was read at 450 nm. All samples were analyzed in duplicate. The standard curve was linear from 8 to 256 ng/mL; serum samples with concentrations higher than 256 ng/mL were diluted.
Intergroup comparisons were performed by using the Mann-Whitney U test. Correlation between parameters was estimated by the Pearson correlation coefficient method. Logistic regression analysis was performed to determine the efficacy of soluble syndecan-1 as a predictor of response to treatment and mortality. Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used to assess intergroup differences. P-values less than 0.05 were considered statistically significant.
The median baseline levels of soluble syndecan-1 in the peripheral blood of patients with MM was 265 ng/mL (range, 98-1,049 ng/mL), whereas that in the normal controls was 81 ng/mL (range, 27-192 ng/mL); this difference was statistically significant (P <0.0001). The soluble syndecan-1 levels in 21 patients (75%) were higher than the mean level + 2 SD (>162 ng/mL) of control group (Fig. 1). The median soluble syndecan-1 level in the BM of the control group patients was 563 ng/mL (range, 211-2,389 ng/mL), and the soluble syndecan-1 levels in the BM correlated with those in the blood (r=0.86; P=0.02).
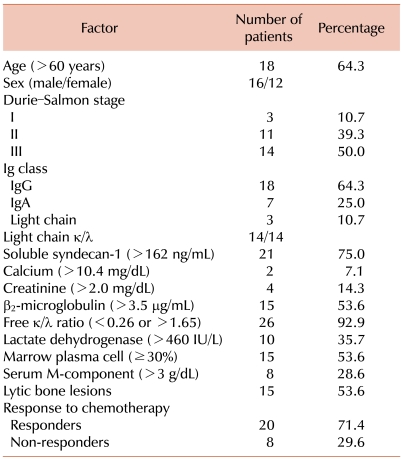
The clinical characteristics of the 28 MM patients are shown in Table 1. According to the Durie-Salmon staging system, 3 patients (10.7%) were graded as stage I MM; 11 patients (39.3%), stage II MM; and 14 patients (50%), stage III MM. Intergroup differences with regard to the median soluble syndecan-1 levels in patients with different MM stages were significant (P=0.045). The median soluble syndecan-1 level in patients with stage I/II MM was 186.5 ng/mL (range, 98-785 ng/mL) and that in patients with stage III MM was 349 ng/mL (range, 101-1,049 ng/mL).
The distribution of immunoglobulins in the patients was as follows: IgG, 64.3% (n=18) and IgA, 25% (n=7); 3 patients had light-chain disease (10.7%). The soluble syndecan-1 levels in patients with IgG (median level, 264.5 ng/mL; range, 98-528 ng/mL) were not different from those in patients with other immunoglobulins (median level, 269 ng/mL; range, 101-1,049 ng/mL). Lytic bone lesions were observed in 15 patients, and the median soluble syndecan-1 levels in the patients with lytic bone lesions was not significantly different from those in the patients without lytic bone lesions (306 ng/mL vs. 257 ng/mL; P=0.76). Chromosomal abnormalities were observed in 4 patients (complex karyotype, 3 patients and marker chromosome, 1 patient), and the median soluble syndecan-1 levels in patients with normal karyotypes were not significantly different from those in patients with abnormal karyotypes (P=0.36).
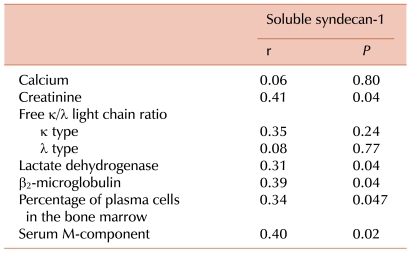
Soluble syndecan-1 levels positively correlated with other parameters such as percentage of plasma cells in the BM (r=0.34; P=0.047), β2 microglobulin level (r=0.39; P=0.04), and serum M-protein concentration (r=0.40; P=0.02). In addition, soluble syndecan-1 levels were clearly correlated with creatinine (r=0.41; P=0.04) and LD (r=0.31; P=0.04) levels. However, soluble syndecan-1 levels did not correlate with the calcium level and free κ/λ light chain ratio (Table 2).
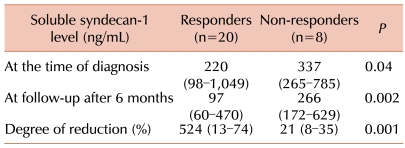
All 7 patients (100%) with low baseline levels of soluble syndecan-1 (≤162 ng/mL) responded to chemotherapy, but only 13 (61.9%) among the 21 patients with high baseline levels of soluble syndecan-1 (>162 ng/mL) showed a therapeutic response. However, the rate of therapeutic response in patients with low baseline levels of soluble syndecan-1 was not significantly different from that in patients with high baseline levels of soluble syndecan-1 (P=0.07). After 6 months, 14 (50%) of the 28 patients showed low levels of soluble syndecan-1. The median soluble syndecan-1 level at the time of diagnosis in the responders was significantly lower than that in the non-responders (P=0.04). The degree of reduction in soluble syndecan-1 levels after 6 months in the responders was greater than that in the non-responders (P=0.001) (Table 3).
However, analysis by univariate logistic regression revealed that soluble syndecan-1 was not a significant predictor of therapeutic response (P=0.56). Other parameters such as serum creatinine level (P=0.13), β2 microglobulin level (P=0.44), serum M-component concentration (P=0.08), and the percentage of plasma cells in the BM (P=0.66) were not significant predictors.
The median follow-up time for all patients was 25.5 months (range, 7.5-43 months). During follow-up, 5 patients died, and 3 patients were lost to follow up. The soluble syndecan-1 levels at the time of diagnosis and 6 months after the diagnosis were not significant predictors of mortality (P=0.36; P=0.26), while the other parameters were not significant.
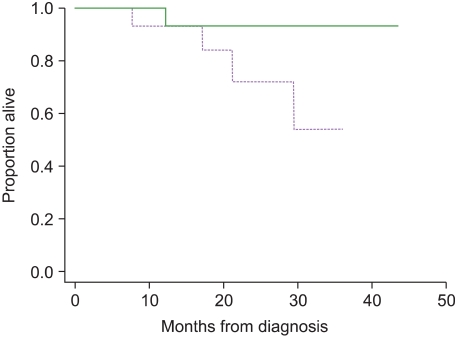
The 3-year overall survival rates in patients with high (>162 ng/mL) and low levels of soluble syndecan-1 at diagnosis were 59% (21 patients; 5 deaths) and 100% (7 patients; no death), respectively; however, the difference between these survival rates was not significant (P=0.11). The 3-year overall survival rates in patients with high and low levels of soluble syndecan-1 after 6 months were 54% (14 patients; 4 deaths) and 93% (14 patients; 1 death), respectively, but the difference between these rates was not significant (P=0.08) (Fig. 2). The 3-year overall survival rates in patients with less and more than 30% reduction in soluble syndecan-1 levels were 65% (10 patients; 3 deaths) and 75% (18 patients; 2 deaths), respectively, but the difference between these rates was not significant (P=0.18).
Syndecan-1 is expressed on mature plasma cells and can exist in the serum as soluble syndecan-1 after being shed from the cell surface. Soluble syndecan-1 is present in low levels in normal populations, but its levels have been reported to be elevated in myeloma patients [4]. In animal studies, high levels of soluble syndecan-1 have been associated with the growth and metastasis of tumors [9]. In this study, the median soluble syndecan-1 level in MM patients (265 ng/mL) was significantly higher than that in the normal controls. The median soluble syndecan-1 level in our study was lower than that (336 ng/mL) reported by Lovell et al. in a recent large study conducted in 324 patients [6], but it was higher than the levels reported by Siedel et al. and Kumar et al. (164 ng/mL and 158 ng/mL, respectively) [5, 10]. The differences in the median levels can be attributed to racial differences and the difference in the proportion of patients with stage III disease in this study (50% stage III), which was different from those reported by Lovell (stage III, 88% patients) and Siedel (stage III, 49% patients).
In our study, soluble syndecan-1 levels were not elevated in all the MM patients, and soluble syndecan-1 levels in 25% of the patients were within the normal range (18-162 ng/mL). Among the 7 patients with normal levels of soluble syndecan-1, 6 patients had stage I or II MM, and only 1 patient had stage III MM. This finding is consistent with those of previous studies, which reported that some myeloma patients had normal levels of soluble syndecan-1 at the time of diagnosis [5, 6, 10], and suggests that the changes in soluble syndecan-1 levels can be attributed to the disease stage. In this study, the sensitivity of soluble syndecan-1 in patients with MM was 75%; therefore, soluble syndecan-1 does not have adequate diagnostic value.
Previous studies have shown that soluble syndecan-1 levels correlated with those of other disease markers, and soluble syndecan-1 levels in myeloma patients were different from those with monoclonal gammopathy of unknown significance [10-13]. Intergroup differences with regard to the median soluble syndecan-1 levels in patients grouped according to the MM stages were significant; soluble syndecan-1 levels in the blood correlated with those in the BM. These results suggest a causal relationship between the blood level of soluble syndecan-1 and tumor mass. However, the presence of abnormal karyotypes and various Ig subtypes did not affect the soluble syndecan-1 levels; although hypodiplody and the presence of non-IgG subtypes were reported to be prognostic factors for MM [14, 15].
Positive correlations have been reported between soluble syndecan-1 levels and those of other prognostic factors and markers of tumor burden [16, 17], such as percentage of plasma cells in the BM, β2 microglobulin level, and serum M-protein concentration. The levels of soluble syndecan-1 have also been reported to be correlated with those of creatinine, which in turn were associated with the severity of renal failure [18]. This finding is consistent with those of previous studies [5, 6, 10, 11] and suggests that soluble syndecan-1 plays a role in myeloma progression and that soluble syndecan-1 level is an indicator of the severity of renal failure. However, predictive value of the levels of soluble syndecan-1 for therapeutic response and mortality has not yet been substantiated.
Dhodapkar et al. [19] showed that the presence of syndecan-1 on the cell surface can counteract bone destruction in murine BM cell cultures. However, in this study, soluble syndecan-1 levels in patients with lytic bone lesions were not different from those without lytic bone lesions and did not correlate with calcium concentration. Therefore, soluble syndecan-1 probably does not affect bone destruction.
Previous studies have shown that the soluble syndecan-1 level at the time of diagnosis is a powerful and independent prognostic factor for multiple myeloma [5, 6, 10]. In particular, the median survival time of patients with high levels of soluble syndecan-1 was lesser than that of patients with low levels of soluble syndecan-1. However, the survival rate of patients with high levels of soluble syndecan-1 was not significantly different from that of patients with low levels of soluble syndecan-1 (P=0.11); this finding may be explained by the smaller number of patients and shorter follow-up period in our study as compared to those in previous studies. In addition, in this study, the survival rate of patients with high levels of soluble syndecan-1 at the time of follow-up examination was lower than the corresponding rate of patients with low levels of soluble syndecan-1, although the difference was not significant (P=0.08). Thus far, no study has examined the relationship between the soluble syndecan-1 levels at the time of follow-up examination and survival. The results of our study suggest that the soluble syndecan-1 level at the time of follow-up examination can be associated with prognosis for MM.
The difference between responders and non-responders with regard to the degree of reduction in soluble syndecan-1 levels 6 months after chemotherapy was significant, and the median soluble syndecan-1 level at the time of diagnosis in the responders was significantly higher than that in the non-responders. However, the frequency of therapeutic response in patients with high levels of soluble syndecan-1 did not differ significantly from that in patients with low levels of soluble syndecan-1 (P=0.14), although all non-responders showed high levels of soluble syndecan-1 at the time of diagnosis. The degree of reduction in soluble syndecan-1 level after 6 months was unrelated to any significant difference in survival rate (P=0.18). We did not statistically prove that a high level of soluble syndecan-1 at the time of diagnosis is associated with a therapeutic response, but the trend showed that the rate of therapeutic response in the patients with low baseline levels of soluble syndecan-1 was higher than that in the patients with high baseline levels of soluble syndecan-1.
In conclusion, the use of soluble syndecan-1 as a tool for diagnosing MM has limitations. However, in this study, the soluble syndecan-1 level correlated with those of other disease markers and had some predictive value for response to chemotherapy. Further studies should be performed to investigate the prognostic value of soluble syndecan-1 levels.
References
1. Costes V, Magen V, Legouffe E, et al. The Mi15 monoclonal antibody (anti-syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens. Hum Pathol. 1999; 30:1405–1411. PMID: 10667416.

2. Sanderson RD, Børset M. Syndecan-1 in B lymphoid malignancies. Ann Hematol. 2002; 81:125–135. PMID: 11904737.

3. Langford JK, Yang Y, Kieber-Emmons T, Sanderson RD. Identification of an invasion regulatory domain within the core protein of syndecan-1. J Biol Chem. 2005; 280:3467–3473. PMID: 15563454.

4. Dhodapkar MV, Kelly T, Theus A, Athota AB, Barlogie B, Sanderson RD. Elevated levels of shed syndecan-1 correlate with tumour mass and decreased matrix metalloproteinase-9 activity in the serum of patients with multiple myeloma. Br J Haematol. 1997; 99:368–371. PMID: 9375756.

5. Seidel C, Sundan A, Hjorth M, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000; 95:388–392. PMID: 10627439.

6. Lovell R, Dunn JA, Begum G, et al. Soluble syndecan-1 level at diagnosis is an independent prognostic factor in multiple myeloma and the extent of fall from diagnosis to plateau predicts for overall survival. Br J Haematol. 2005; 130:542–548. PMID: 16098068.

7. Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975; 36:842–854. PMID: 1182674.

8. Smith A, Wisloff F, Samson D. UK myeloma Forum, Nordic Myeloma Study Group, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006; 132:410–451. PMID: 16412016.

9. Yang Y, Yaccoby S, Liu W, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002; 100:610–617. PMID: 12091355.
10. Kumar S, Blood E, Oken MM, Greipp PR. Prognostic value of syndecan-1 in multiple myeloma and its relationship with other prognostic factors. Blood. 2004; 104(11):2402.

11. Jánosi J, Sebestyén A, Mikala G, Németh J, Kiss Z, Vályi-Nagy I. Soluble syndecan-1 levels in different plasma cell dyscrasias and in different stages of multiple myeloma. Haematologica. 2004; 89:370–371. PMID: 15020284.
12. Kyrtsonis MC, Vassilakopoulos TP, Siakantaris MP, et al. Serum syndecan-1, basic fibroblast growth factor and osteoprotegerin in myeloma patients at diagnosis and during the course of the disease. Eur J Haematol. 2004; 72:252–258. PMID: 15089762.

13. Schaar CG, Vermeer HJ, Wijermans PW, Huisman W, le Cessie S, Kluin-Nelemans HC. Serum syndecan-1 in patients with newly diagnosed monoclonal proteinemia. Haematologica. 2005; 90:1437–1438. PMID: 16219583.
14. Fassas AB, Spencer T, Sawyer J, et al. Both hypodiploidy and deletion of chromosome 13 independently confer poor prognosis in multiple myeloma. Br J Haematol. 2002; 118:1041–1047. PMID: 12199783.

15. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005; 106:812–817. PMID: 15855274.

16. Joshua DE, Brown RD, Gibson J. Prognostic factors in myeloma: what they tell us about the pathophysiology of the disease. Leuk Lymphoma. 1994; 15:375–381. PMID: 7873994.

17. Salmon SE, Smith BA. Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest. 1970; 49:1114–1121. PMID: 4987170.

18. Kyle RA. Prognostic factors in multiple myeloma. Stem Cells. 1995; 13(Suppl. 2):56–63. PMID: 8520513.

19. Dhodapkar MV, Abe E, Theus A, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998; 91:2679–2688. PMID: 9531576.

Fig. 1
Soluble syndecan-1 levels at diagnosis in 28 patients with multiple myeloma. Horizontal line denotes mean +2 SD (162 ng/mL) of 50 healthy controls (sensitivity, 75% and specificity, 94%).

Fig. 2
Kaplan-Meier survival curves for patients classified on the basis of the soluble syndecan-1 levels at 6 months after treatment. The solid line represents patients with low levels of soluble syndecan-1 (≤162 ng/mL), and the dotted line represents patients with high levels of soluble syndecan-1 (>162 ng/mL) (P=0.08).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download