Abstract
Background
A combination of busulfan (Bu) and cyclophosphamide (Cy) has been used as a standard myeloablative regimen for allogeneic hematopoietic stem cell transplantation (HSCT). Recent studies postulate that fludarabine (Flu) is a less toxic substitute for Cy.
Methods
Forty-two patients who were diagnosed with acute leukemia or myelodysplastic syndrome and received BuFlu (n=17) or BuCy (n=25) from August, 1999 to July, 2009 at Dong-A University Medical Center were retrospectively analyzed.
Results
The median follow-up duration was 39.75 months. The BuFlu group showed a lower incidence of mucositis (P=0.005), but there was no significant intergroup difference in the time of engraftment, nausea/vomiting, acute/chronic graft-versus-host disease, hepatic veno-occlusive disease, or hemorrhagic cystitis. Moreover, the 2 groups showed no significant difference in the cumulative risk of relapse, event-free survival, or overall survival.
Since the early 1980s, busulfan (Bu) has been recognized as an effective pretransplant agent in lieu of total body irradiation in the conditioning therapy for allogeneic hematopoietic stem cell transplantation (HSCT). Thus, both oral and intravenous (i.v.) forms of Bu have been used in combination with cyclophosphamide (Cy), and this combination has become a standard myeloablative regimen for allogeneic HSCT [1, 2]. However, these commonly used transplant preparative regimens cause a spectrum of acute and chronic toxicities: nausea/vomiting, oral mucositis, enteritis, hepatic veno-occlusive disease (HVOD), acute graft-versus-host disease (aGVHD), and chronic GVHD (cGVHD) [3]. Both the porto-hepatic metabolism of oral Bu [4] and the specific metabolite of Cy [5] are highly associated with HVOD, increased treatment-related mortality (TRM), and morbidity. However, i.v. Bu administration is associated with a significantly lower incidence of HVOD because of the predictable bioavailability in this route of administration and the circumvention of the first-pass effect of oral administration [6].
Fludarabine (Flu), a purine analog, has been shown to be active against a variety of hematologic malignancies [7]. In addition, by inhibiting lymphocyte proliferation, Flu provides sufficient immunosuppression to prevent graft rejection. In the light of its established characteristics and non-hematological toxicities, Flu has been used in nonmyeloablative transplant settings [8]. Some studies have shown that the use of Flu in reduced-intensity conditioning regimens enabled engraftment, promoted the graft-versus-leukemia effect, and was well tolerated by the patients [9-11]. Flu has also been combined with myeloablative doses of Bu (BuFlu) [12-14], and this combination showed a lower rate of complications, successful engraftment, and efficacy in patients with a high risk of leukemia and in middle-aged patients with related and unrelated allogeneic HSCT. Available retrospective data comparing BuFlu with BuCy suggest that BuFlu is safe and at least as effective as BuCy for patients who have myelogenous malignancies and are undergoing HSCT [15, 16].
In this study, we have retrospectively analyzed patients who had acute leukemia or myelodysplastic syndrome (MDS) and underwent allogeneic HSCT after myeloablative conditioning regimens using BuFlu. We compared the data of these patients with those who received the traditional BuCy regimen to compare their toxicity profiles, treatment outcomes, and overall survival (OS).
From August, 1999 to July, 2009, 42 patients who were diagnosed with acute leukemia or MDS underwent HLA-identical allogeneic HSCT at Dong-A University Medical Center. The findings for 15 patients who received oral BuCy and 10 patients who received i.v. BuCy were compared to those for 17 consecutive patients who were treated with the myeloablative BuFlu regimen. All patients were retrospectively analyzed by reviewing their medical records. We have excluded patients with chronic myeloid leukemia or aplastic anemia because they had better clinical outcomes than patients with acute leukemia or MDS after allogeneic HSCT.
In the BuCy group, oral Bu (Myran, Korea United Pharm., Chungnam, Republic of Korea, 1 mg/kg) or i.v. Bu (Busulfex, Ben Venue Laboratories Inc., Bedford, Ohio, USA, 0.8 mg/kg) was administered every 6 hours for 4 days (days -7 to -4), followed by i.v. Cy (60 mg/kg) for 2 days (days -3 to -2). The chemotherapy doses were based on the ideal body weight (IBW), except in the patients whose real body weight (RBW) exceeded their IBW by more than 20%; in such cases, the doses were based on an adjusted IBW, which was calculated as IBW + (0.25 (RBW - IBW)).
In the BuFlu group, i.v. Flu (Fludara, BaxterOncology GmbH, Westfalen, Germany) was administered at a dose of 40 mg/m2 over 30 min for 4 days (total, 160 mg/m2, days -6 to -3) along with i.v. Bu at a dose of 130 mg/m2 over 3 hours for 4 days (total, 520 mg/m2, days -6 to -3).
The aGVHD prophylaxis consisted of methotrexate and cyclosporin A. Cyclosporin A administration was started at a dose of 1.5 mg/kg i.v. over 3 hours q 12 hours from days -1 to +14 and then changed to the oral form at a dosage of 4-6 mg/kg bid adjusted after therapeutic drug monitoring at a level of 200-400 ng/mL. Patients with unrelated donors in the BuFlu group received rabbit-ATG (Thymoglobulin, IMTIX-SANGSTAT, Lyon, France) at a dose of 2.0 mg/kg i.v. from days -3 to -1. Methotrexate was administered at doses of 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11. The last dose of methotrexate was omitted when mucositis (≥grade 4) or renal impairment was observed. Phenytoin was administered during and 1 day after i.v. Bu-based therapy. Allogeneic donor hematopoietic stem cells were infused using the standard infusion technique on day 0.
Infection prophylaxis consisted of a combination of ciprofloxacin (500 mg p.o. bid), fluconazole (100 mg/day p.o. qd), acyclovir (250 mg/m2 i.v. q 8 hours), and sulfamethoxazole-trimethoprim (960 mg p.o. 3 times a week); this prophylaxis began with the initiation of conditioning. Ursodeoxycholic acid (100 mg p.o. tid) and heparin (100 units/kg/day i.v. continuously) were used for HVOD prophylaxis, which also began with the initiation of the conditioning therapy. A CMV antigenemia assay was performed every week until day +100, every 2 weeks until 6 months, and every 2 or 4 weeks until 12 months after engraftment.
Successful neutrophil engraftment was defined as the first of 2 consecutive days with an absolute neutrophil count ≥0.5×109/L. Failure to engraft in the absence of malignancy by day +30 was considered primary engraftment failure. Secondary graft failure was defined as initially successful engraftment with documented donor-derived hematopoiesis followed by loss of graft function without recurrent malignancy. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count ≥20×109/L without transfusion.
aGVHD was defined as described by Przepiorka et al. [17], and cGVHD was defined as described by the "Revised Seattle Criteria" [18]. HVOD was graded according to the criteria by McDonald et al. [19]. Toxicity was scored using the modified National Cancer Institute criteria (CTC 3.0). The event-free survival (EFS) time was defined as the time from transplantation to relapse or death, and the OS was defined as the number of days from transplantation until death from any cause; in contrast, non-relapse mortality (NRM) was defined as death from any cause other than disease relapse.
Categorical variables and continuous variables were compared by Fisher's exact test and t test, respectively. OS and EFS were estimated using the Kaplan-Meier product limit method. Cumulative incidence of relapse was estimated by Gray's test. The Cox proportional hazard regression model was employed in univariate and multivariate analyses of OS and EFS. Calculation of adjusted P-values was performed by the backward selection method. This study was essentially explorative in nature, and therefore, no adjustment for multiple testing was applied, and P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.1.3 and R 2.9.1 statistical software.
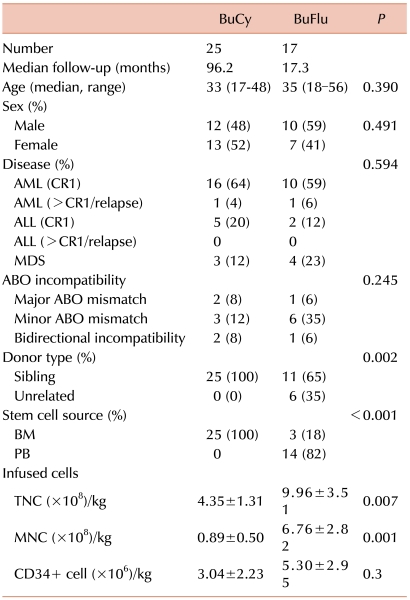
Among the 42 patients who were analyzed in this study, 25 received BuCy and 17 received BuFlu as preparative conditioning therapy. Baseline patient characteristics are listed in Table 1. The median patient age was 34 years (range, 17-56 years). All recipients were matched with HLA-identical donors, among whom 6 donors (14%) were unrelated. Twenty-eight patients underwent bone marrow transplantation (BMT). Peripheral blood stem cell transplantation (PBSCT) was conducted in 14 patients. The mean number of infused total nucleated cells (TNCs)/kg, mononucleated cells (MNCs)/kg, and CD34+ stem cells/kg were 6.46±3.61 (×108)/kg, 3.05±3.34 (×108)/kg, and 3.95±2.75 (×106)/kg, respectively. The median follow-up duration was 39.75 months (range, 2.70-127.10 months). There were no intergroup differences in age, sex, disease types of the recipients, ABO incompatibility status, or number of infused CD34+ stem cells. However, stem cell sources (P<0.001), donor types (P=0.002), and total number of infused stem cells (TNCs, P=0.007; MNCs, P=0.001) showed statistically significant differences. All patients in the BuCy group received stem cells from sibling donors and were infused with 4.35±1.31 (×108/kg) TNCs and 0.89±0.50 (×108/kg) MNCs. However, in the BuFlu group, 6 patients received stem cells from unrelated donors, and the remaining 11 patients received stem cells from sibling donors; 14 patients underwent PBSCT, and 3 patients received BMT. Among the 6 patients who underwent HSCT from unrelated donors, 3 patients each underwent PBSCT and BMT. Patients in the BuFlu group were infused with TNCs [mean, 9.96±3.51 (×108/kg)] and MNCs [mean, 6.76±2.82 (×108/kg)].
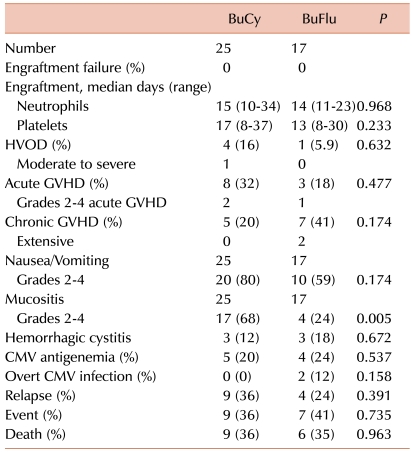
All 42 patients receiving BuCy or BuFlu showed successful engraftment (Table 2). No primary engraftment failure or secondary graft failure was observed in either group. There were no statistically significant intergroup differences in the time to neutrophil engraftment (14.0 vs. 15.0 days, P=0.968) or platelet engraftment (13.0 vs. 17.0 days, P=0.233) after transplantation.
Four cases of HVOD were observed in the BuCy group. Among these, 3 patients, including 1 patient who showed moderate HVOD, were treated with oral Bu. In the BuFlu group, 1 patient experienced mild HVOD. There was no significant difference between the number of HVOD cases in the 2 groups (P=0.632).
aGVHD developed in 8 patients (32%) in the BuCy group. Among these, 6 patients (75%) showed grade 1 aGVHD and 2 patients treated with oral Bu showed grade 3 aGVHD. Three patients (18%) showed aGVHD in the BuFlu group. In this group, 2 patients showed grade 1 aGVHD and 1 patient showed grade 2 aGVHD. There was no statistically significant difference between the number of cases of aGVHD in the 2 groups (P=0.477). There were no cases of grade 4 aGVHD in the 2 groups.
cGVHD developed in 5 patients (20%) in the BuCy group and in 7 patients (41%) in the BuFlu group. Extensive cGVHD developed in 2 patients in the BuFlu group, but no patient showed extensive disease in the BuCy group. There was no significant difference between the number of cases of cGVHD in the 2 groups (P=0.174).
All patients experienced nausea, vomiting, and mucositis. Twenty patients (80%) in the BuCy group and 10 (59%) patients in the BuFlu group showed grade 2-4 nausea/vomiting. Among the 20 patients in the BuCy group, 17 were treated with oral Bu. There was no significant difference between the incidence of these symptoms in the 2 groups (P=0.174).
Grade 2-4 mucositis developed in 17 patients (68%) in the BuCy group and in 4 patients (24%) in the BuFlu group. There was a significant difference between the number of cases showing mucositis in the 2 groups (P=0.005).
Hemorrhagic cystitis developed in 3 patients (12%) in the BuCy group and in 3 patients (18%) in the BuFlu group. There was no significant difference between the 2 groups (P=0.672).
CMV antigenemia was detected in 5 patients (20%) in the BuCy group and in 4 patients (24%) in the BuFlu group. Two patients in the BuFlu group experienced CMV pneumonia. There was no significant difference in the occurrence of CMV antigenemia between the 2 groups (P=0.537).
Three patients (7%) died for reasons not related to relapsed or refractory disease. All of these patients were treated with the BuFlu regimen and diagnosed with MDS. One patient died of septic shock due to delayed hospital arrival, and 2 patients died of CMV pneumonia.
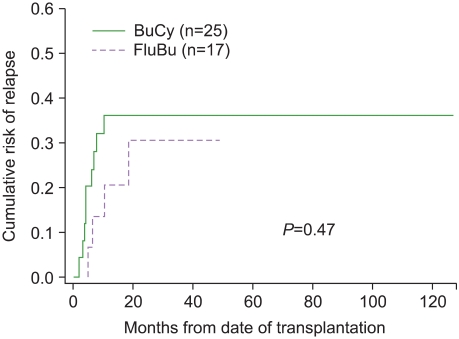
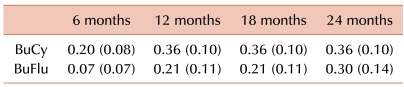
Among the patients who underwent allogeneic HSCT, 13 (31%) relapsed; 9 (36%) patients were treated with BuCy and 4 (24%) with BuFlu. The cumulative risk of relapse at 12 months and 24 months after transplantation were respectively 36% (s.e.=10%) and 36% (s.e.=10%) in the BuCy group and 21% (s.e.=11%) and 30% (s.e.=14%) in the BuFlu group (Table 3). No significant difference in the cumulative risk of relapse was observed between the 2 groups (P=0.47) (Fig. 1).
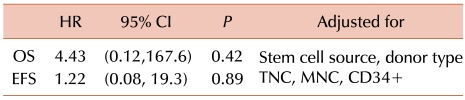
There were no significant intergroup differences in OS and EFS (P=0.86 and P=0.79, respectively) (Fig. 2). In the BuCy group, the 3-year OS and EFS were 64% (s.e.=10%) and 64% (s.e.=10%), respectively. In the BuFlu group, the 3-year OS and EFS were 58% (s.e.=13%) and 55% (s.e.=13%), respectively. In the univariate analysis, none of the variables was found to affect OS and EFS. The multivariate analysis showed that the TNC count was associated with good OS and EFS (HR=0.59; 95% CI, 0.40-0.88; P=0.009 and HR=0.69; 95% CI, 0.50-0.96; P=0.026, respectively), whereas the CD34+ cell count was marginally associated with poor OS (HR=1.34; 95% CI, 1.01-1.78; P=0.045). The adjusted P=values for stem cell source, donor type, and number of infused cells are shown in Table 4.
The combination regimen of oral and i.v. Bu and Cy has been widely accepted as a standard preparative conditioning therapy in allogeneic HSCT. However, the highly cytotoxic effects elicited by combining these 2 alkylating drugs increase the TRM and morbidity in patients who undergo this regimen. The cytotoxicity of Cy is putatively caused by the initial conversion of Cy to 4-hydroxycyclophosphamide (HCY), a circulating metabolite that is thought to enter the target cells. This conversion reaction is catalyzed by peroxidases and cytochrome P450. Exposure to HCY is modulated by Bu and/or phenytoin, and individuals may show substantial variability in exposure to HCY at a given dose of Cy [20]. Therefore, the cytotoxicity of the BuCy conditioning regimen affects single or multiple organs and ranges from mild to severe, eventually threatening the patients' lives. Moreover, oral Bu administration in combination with Cy has been associated with HVOD. Additionally, oral Bu administration is associated with a hepatic first-pass extraction effect that can result in high local Bu concentrations in the portal-hepatic venous system, which may conceivably contribute to the development of HVOD [21].
To alleviate the oral Bu and Cy toxicities, i.v. Bu formulation and an alternative immunosuppressive agent bypassing the hepatic metabolism, that is, replacing Cy with a nucleoside analog, was introduced [21]. We changed the conditioning regimen at our institution to decrease the toxicity and increase the convenience of treatment because the long half-life of Flu allows once-daily administration.
Flu performs an immunosuppressive role, thereby creating an environment that promotes donor stem cell engraftment. Moreover, Flu indirectly but synergistically enhances Bu-induced cytotoxicity by interfering with the repair of radiation therapy (XRT)- and alkylator-induced DNA damage [7]. However, the superiority of the BuFlu (compared to the BuCy) conditioning regimen in terms of the disease relapse rate remains controversial because a lower incidence of toxicity implies lesser cytotoxic effects of the drug.
In our study, patients who were treated with the BuFlu regimen conclusively benefited from the treatment with regard to their oral mucositis grade. However, 15 patients (60%) in the BuCy group received oral Bu, but all patients in the BuFlu group received i.v. Bu. This difference may affect the statistical significance because of the unpredictable and erratic bioavailability of the orally administered drug.
Although the number of patients was too small to generalize the results of our study, the BuFlu group showed a tendency of faster platelet engraftment and lower tendencies to develop HVOD, grade 2-4 aGVHD, nausea, and vomiting in comparison with the corresponding values for the BuCy group. The relatively shorter time to platelet engraftment in the BuFlu group could be attributed to Flu itself, which has less cytotoxic and similar immunosuppressive effects than Cy, thereby allowing more easy incorporation of the donor's stem cells into the recipients' bone marrow. However, most of the analyzed patients in the BuFlu group received stem cells from peripheral blood, which may have partially contributed to the rapid engraftment. As mentioned above, aGVHD and HVOD primarily occur due to the tissue damage caused by cytotoxic agents used in preparative conditioning regimens. Flu has a less direct toxic effect on the host environment, thereby lowering TRM and morbidity. However, more patients in the BuFlu group experienced cGVHD. This might have been due to the peripheral blood stem cells, which comprised the most significant stem cell source in this group. PBSCT has a higher incidence of cGVHD because the number of T lymphocytes in the peripheral blood is more than that in the bone marrow [22].
In this study, both the BuCy and BuFlu groups showed similar rates of CMV antigenemia. Nevertheless, the patients in the BuFlu group showed a higher, although not significant, incidence of overt CMV disease, thereby resulting in a higher incidence of non-relapse mortality in this group. Although the number of patients was too small to generalize our results, Flu could be more immunosuppressive than Cy when incorporated into a conditioning regimen with Bu, thereby necessitating more caution with respect to serious infections.
The lower cytotoxicity of Flu could imply a lower antitumor effect, which might induce a higher relapse rate and shorter survival time. In our study, patients in both groups showed no difference in disease recurrence or OS, as had been demonstrated in several studies using BuFlu [12-14]. Although these results are not conclusive because of the small number of patients analyzed and the shorter follow-up duration in the BuFlu group, we concluded that Flu combined with Bu could be an effective conditioning regimen, in lieu of the BuCy regimen, with fewer adverse events.
In this study, we used a sequential infusion regimen of Flu and Bu for 4 days [12]. Various doses of Flu have been used in the BuFlu regimen for reduced-intensity or myeloablative conditioning. Although some controversies exist, the doses of Flu for myeloablative conditioning range from 120 to 250 mg/m2 [12-14]. A higher dose of Flu results in a lower occurrence rate of GVHD but a longer time to engraftment [12, 14, 23]. However, the optimal sequence, infusion timing, or doses of Bu and Flu for a myeloablative conditioning regimen have not been established yet.
In conclusion, the only significant benefit of the BuFlu regimen was the lower incidence of oral mucositis. There was no significant intergroup difference in the toxicity profiles, including the incidence of HVOD, aGVHD, cGVHD, nausea/vomiting, hemorrhagic cystitis, or CMV antigenemia, although patients in the BuFlu group tended to show shorter platelet engraftment times and lower incidence of HVOD, aGVHD, and nausea/vomiting. We observed no significant difference in the EFS and OS of the 2 groups. Patients in the BuFlu group showed a tendency toward a higher incidence of severe CMV infections. Thus, Flu combined with Bu could be incorporated in a myeloablative conditioning regimen for allogeneic HSCT with an efficacy similar to that of Cy combined with Bu, but more caution is needed because the combination of Flu and Bu can induce fatal infections. Nevertheless, the results of our study have limited reliability in establishing a general consensus. First, the number of patients was too small, and the baseline donor stem cell types and sources of stem cells showed significant differences between the 2 groups. Second, Flu could have different pharmacokinetics, consequently resulting in different efficacies in various diseases. Third, and of primary concern, the different conditioning regimens were administered sequentially - oral BuCy was administered from 1999-2002, i.v. BuCy from 2002-2005, and BuFlu from 2005-2009, thereby resulting in different follow-up durations and "period effects" between the groups. The difference in the treatment time can affect the therapeutic results by confounding variables unrelated to the preparative conditioning regimen. Fourth, a selection bias exists in our study; all patients who had been treated with BuCy underwent allogeneic HSCT with related donor bone marrow; in contrast, most patients in the BuFlu group received peripheral blood stem cells. In addition, some patients in the BuFlu group received unrelated donor stem cells. Thus, randomized prospective studies in large populations comparing the BuCy and BuFlu regimens are needed.
References
1. Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996; 37:401–408. PMID: 8599861.

2. Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987; 70:1382–1388. PMID: 3311203.

3. de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004; 104:865–872. PMID: 15090449.

4. Peters WP, Henner WD, Grochow LB, et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res. 1987; 47:6402–6406. PMID: 2824032.
5. McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003; 101:2043–2048. PMID: 12406916.

6. Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: Decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002; 8:493–500. PMID: 12374454.

7. Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002; 41:93–103. PMID: 11888330.

8. Hillmen P. Future prospects for fludarabine-containing regimens in the treatment of hematological cancers. Hematol J. 2004; 5(Suppl 1):S76–S86. PMID: 15079156.

9. Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997; 89:4531–4536. PMID: 9192777.

10. Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001; 97:631–637. PMID: 11157478.

11. Khouri IF, Keating M, Körbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998; 16:2817–2824. PMID: 9704734.

12. de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004; 104:857–864. PMID: 15073038.

13. Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003; 102:820–826. PMID: 12676781.
14. Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002; 8:468–476. PMID: 12374451.

15. Chae YS, Sohn SK, Kim JG, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant. 2007; 40:541–547. PMID: 17637692.

16. Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008; 14:672–684. PMID: 18489993.

17. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15:825–828. PMID: 7581076.
18. Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991; 28:250–259. PMID: 1887253.
19. McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993; 118:255–267. PMID: 8420443.

20. Slattery JT, Kalhorn TF, McDonald GB, et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol. 1996; 14:1484–1494. PMID: 8622062.

21. Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009; 15:523–536. PMID: 19361744.

22. Schmitz N, Beksac M, Hasenclever D, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002; 100:761–767. PMID: 12130483.

23. Chunduri S, Dobogai LC, Peace D, et al. Comparable kinetics of myeloablation between fludarabine/full-dose busulfan and fludarabine/ melphalan conditioning regimens in allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006; 38:477–482. PMID: 16980995.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download